Partners Asthma Center Grand Rounds
Joshua A. Boyce, M.D.
The Spectrum of Eosinophilic Pneumonias Part 1
Eosinophilic pneumonias are among the most intriguing clinical problems that allergists and pulmonologists are asked to address. They are a group of rare syndromes held together by the common thread of prominent pulmonary eosinophilia (eosinophils accumulating in the distal air spaces and small airways, producing dysfunction). Distinctive features often allow one to segregate them into discrete syndromes. In this discussion I will endeavor not only to enumerate the individual syndromes grouped under the heading of eosinophilic pneumonia but also to provide a framework to think about them logically from a diagnostic viewpoint. In addition, we will touch upon the basic mechanisms that underlie pulmonary eosinophilia and the implications of these mechanisms for future drug design.
Eosinophil Biology
The eosinophil is a powerful inflammatory effector cell, probably originally specialized to fight helminthic parasitic infections. When an eosinophil meets an antibody-coated parasite, a powerful sequence of events ensue, including a respiratory burst, release of granule proteins, generation of various cytokines, and synthesis of cysteinyl leukotrienes. In the eosinophilic pneumonias, granule proteins such major basic protein, eosinophil cationic protein, and other proteases elicit epithelial cell exfoliation and injury. Free proteins can be recovered in abundance in the bronchoalveolar lavage fluid of patients with eosinophilic pneumonia. It was the presence of these cationic proteins packed within cytoplasmic granules with their avidity for negatively charged stains like eosin that allowed Erlich in the 19th century to first identify this subset of white blood cell.
High-grade peripheral blood eosinophilia occurs in a limited number of disease processes. This finding therefore implies that specific mechanisms must be involved in the development of this eosinophilic response, mechanisms – for instance – that do not involve the neutrophil. The mechanisms first must promote peripheral blood eosinophilia and then tissue eosinophilia. Eosinophils develop in the bone marrow from uncommitted precursors under the influence of two cytokines, interleukin (IL)-3 and granulocyte-macrophage colony stimulating factor (Gm-CSF). These cytokines are not lineage selective; they expand all granulocyte lines. The eosinophil precursor that evolves is a shared precursor for both eosinophils and basophils, explaining why these two cell lines are often found together in elevated numbers, for instance in parasitic infections.
Terminal differentiation of eosinophils, which probably takes place before these cells leave the bone marrow, requires IL-5. IL-5 is also needed for the selective mobilization of mature eosinophils out of the marrow and into the peripheral blood. IL-5 is crucial to the development of peripheral eosinophilia. In most human diseases involving a selective eosinophilia, it is likely that IL-5 is being generated somewhere in the body.
Where IL-5 is being produced influences the tissue localization of the eosinophils. In eosinophilic pneumonias, IL-5 is made in the lung primarily by T lymphocytes and to some extent by mast cells. IL-5 diffuses into the serum and acts like a hormone, effecting terminal differentiation and then release of the eosinophil from the bone marrow.
Peripheral blood eosinophilia per se does not cause disease; in order to create pathology the eosinophil needs to enter into the tissue. For this migration to take place, the eosinophil must sense a chemoattractive signal. Cytokines important in promoting selective eosinophil migration include IL-5 and eotaxin. Both of theses cytokines bind at a single receptor, called CC chemokine receptor 3 or CCR3. CCR3 is highly expressed on eosinophils and basophils but not neutrophils.
As the eosinophil is drawn toward the vascular endothelial surface, an interaction takes place between adhesion molecules expressed on its cell surface and counter ligands found on the suface of endothelial cells. The adhesion molecule expressed on the surface of eosinophils (alpha 4/beta 1 integrin, also known as very late antigen 4 [VLA4]) binds to vascular cell adhesion molecule 1 (VCAM 1). Like CCR3, VLA4 is expressed uniquely by eosinophils and not neutrophils. As a result, it is a suitable target for selective inhibitory therapy, and indeed small molecular weight inhibitors of VLA4 are currently undergoing clinical trials in humans.
Cellular adhesion in the capillaries allows the eosinophil to migrate between adjacent endothelial cells, traveling out of the circulation and into the target tissue. Within the tissue, eosinophils might ordinarily die, undergoing apoptosis (programed cell death) in a matter of hours. However, if bathed in a hospitable environment containing the proper cytokines, especially IL-5, the eosinophil can survive for as long as several weeks. These same cytokines also markedly increase the capacity for cytotoxicity of the eosinophil, activating the cell and promoting dispersal of its granule proteins. The eosinophil thus becomes transformed into a cell capable of causing disease.
Case Example:
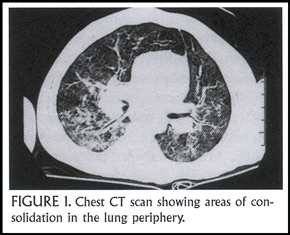
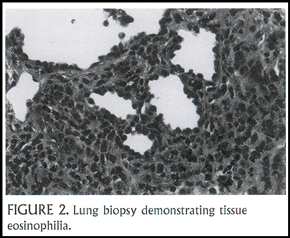
A 2 ½ year-old boy presented with cough and progressive tachypnea. He had peripheral blood eosinophilia (white blood cell count = 11,200 with15% eosinophils). His chest X-ray showed diffuse pulmonary infiltrates, which on chest CT scan were seen to include areas of consolidation in the lung periphery (Figure 1). He underwent a lung biopsy and bronchoalveolar lavage (BAL). The BAL fluid had a high concentration of eosinophils (normally <1%), and the lung biopsy revealed both an eosinophilic alveolitis and bronchiolitis (Figure 2). After a month of treatment with systemic corticosteroids, his chest X-ray cleared entirely.
With the help of Drs. Kathleen Haley and Craig Lilly at the Brigham, we assayed the child’s lung biopsy specimen for expression of some of the cytokines important in eosinophil recruitment and activation. We compared the results with findings in fetal and adult lung samples, neither of which had the presence of eosinophilia. We found evidence (by expression of messenger RNA) for eotaxin 1 in all three specimens and for Gm-CSF in the adult lung tissue. What distinguished the child’s lung from the other samples was the large amount of messenger RNA for IL-5. It is likely that IL-5 production in the lung tissue was the mechanism that led to his eosinophilic pneumonia.
The Eosinophilic Pneumonias: Terminology
The terms eosinophilic pneumonia and pulmonary eosinophilia are sometimes used interchangeably, but it is incorrect to do so. Pulmonary eosinophilia is a pathologic description, referring to the microscopic finding of eosinophils in alveolar walls and alveolar spaces, the interstitial compartment, and distal airways. Eosinophilic inflammation is often accompanied by infiltration with histiocytes and lymphocytes. The finding of pulmonary eosinophilia is not restricted to the eosinophilic pneumonias. It can be seen as a feature of systemic inflammatory diseases, pulmonary infections, and hypersensitivity reactions.
Two specific infectious causes of pulmonary eosinophilia are worth highlighting: strongyloidiasis and tuberculosis. When encountering a patient with pulmonary eosinophilia, it is important to take a detailed history regarding travel and infectious exposures. Strongyloides is endemic in southeastern United States as well as in other tropical climates. The parasite can cause indolent infection of the gastrointestinal tract, and during periods of immunosuppression (such as during treatment with systemic corticosteroids), potentially fatal hyperinfection, including disseminated pulmonary infection, may develop. Tuberculosis rarely causes pulmonary eosinophilia but when it does, it can mimic one of the eosinophilic pneumonias. It too can be made worse by systemic steroid therapy. It is important to remember that eosinophilic pneumonias are not the only cause of pulmonary eosinophilia.
Pulmonary infiltrates with eosinophilia (that is, peripheral blood eosinophilia) or PIE syndrome is another expression used to describe this group of diseases. It suffers from the inaccuracy that often eosinophilic pneumonias will be accompanied by normal peripheral blood eosinophil counts. If you make pulmonary infiltrates with peripheral blood eosinophilia your criteria for exploring the possibility of eosinophilic pneumonia, you will miss some of these cases.
Classification of eosinophilic pneumonias
Eosinophilic pneumonias constitute a small group of etiologically heterogeneous and clinically distinctive diseases in which pulmonary eosinophilia is the unifying feature. A variety of classification systems for the eosinophilic pneumonias have been proposed, with the most widely accepted being that of Liebow and Carrington, proposed in 1969 (See table below).
Table:
|
|
Syndrome |
Agent |
| Leoffler's syndrome (migratory pulmonary infiltrates; duration of illness <1 month |
Helminthic parasitic infection Drugs Unknown |
| Eosinophilic pneumonia complicating chronic asthma |
Drugs Unknown |
| Chronic eosinophilic pneumonia | Helminthic parasitic infection Aspergillus (hypersensitivity; mucoid impaction) Unknown |
| Tropical eosinophilic pneumonia | Filariasis ?Dirofilaria and other zoonoses Unknown |
| Angiitis and eosinophilic pneumonia | Drugs Unknown |
Adapted from: Liebow AA, Carrington CB. The eosinophilic pneumonias. Medicine 1969; 48:251-85 |
|
I find it helpful to think of the eosinophilic pneumonias in two broad groups. In the first group (primary eosinophilic pneumonias), eosinophilic pneumonia is the predominant manifestation of disease and respiratory symptoms are what bring the patient to medical attention. Examples include Loeffler’s syndrome (a transient and generally benign pulmonary eosinophilia usually caused by infection with helminths such as Ascaris lumbricoides), chronic eosinophilic pneumonia, and acute eosinophilic pneumonia. In the second group of eosinophilic pneumonias, pneumonia is a prominent but not exclusive feature of the disease. Examples include Churg-Strauss syndrome, allergic bronchopulmonary aspergillosis, and tropical eosinophilia.
Case example
A six year-old girl presented to the hospital with cough of approximately one month’s duration. She had gradually increasing breathlessness and fevers of increasing intensity. On presentation to the emergency department she appeared ill, was febrile to 102° F, and had a leukocytosis without eosinophilia. Her chest X-ray revealed peripherally based infiltrates in the left lower lobe and right upper lobe.
She was admitted to the hospital and treated with antibiotics for presumed bacterial pneumonia, without improvement. One day wheezes were heard on examination and, as if by reflex, corticosteroids were administered. With systemic steroids her symptoms improved dramatically, her chest X-ray cleared, and she was discharged home.
Approximately one year later she returned to the emergency department with a similar history: gradual onset of cough, progressive dyspnea evolving over a period of about a month, fever, elevated white count, and no peripheral blood eosinophilia. Her chest X-ray again had infiltrates in the lung periphery, again involving the right upper lobe and to some extent the left lower lobe as well. In retrospect, the diagnosis seems obvious, but at the time she underwent open lung biopsy. The lung tissue showed alveolar spaces absolutely packed with lymphocytes, histiocytes and eosinophils. The eosinophils, comprising approximately 30% of the inflammatory cell infiltrate, were in various stages of degranulation. The clinical history, chest radiographs, and lung histopathology are typical of chronic eosinophic pneumonia.
Primary Eosinophilic PneumoniaS
Chronic eosinophilic pneumonia
Chronic eosinophilic pneumonia was first described by Christofordus and Molinar in 1960, but the disease was brought to medical attention by the report of Carrington and colleagues published in the New England Journal of Medicine in 1969. Chronic eosinophilic pneumonia is a distinctive syndrome typically characterized by the insidious onset of cough, fever, dyspnea and progressive weight loss in adults. Commonly, but not always, the patient has a pre-existing diagnosis of asthma. It occurs in women more often than men with a 2-1 female predominance. The most distinctive feature is the radiographic appearance of dense, peripherally-distributed alveolar infiltrates, described as having the appearance of the photographic negative of pulmonary edema on chest X-ray. Only approximately one quarter of all cases of biopsy-proven cases of chronic eosinophilic pneumonia have this classic radiographic appearance, although the percentage probably increases with the use of chest CT scanning, which more readily demonstrates this predilection for eosinophil accumulation in the lung periphery.
Pulmonary function tests tend to show a mixture of obstruction and restriction. Peripheral white blood cell counts usually reveal eosinophilia, but, as in this example, peripheral eosinophilia may be absent. Serum immunoglobulin E (IgE) is elevated in approximately 2/3 of patients, and the sedimentation rate is usually elevated.
Lung pathology sometimes reveals a low-grade, small-vessel vasculitis, suggesting in some cases overlap with Churg-Strauss syndrome. On BAL, IL-5 can be retrieved in high concentrations from involved segments but not in adjacent, uninvolved lung segments. Curiously, recurrences typically involve the same lung areas as the initial involvement. Chronic eosinophilic pneumonia is exquisitely steroid-responsive, but frequent recurrences often make prolonged therapy necessary. The patient whom I presented is now 17 years old and perfectly healthy. She has been off steroids for six years.
Acute eosinophilic pneumonia
In contradistinction to chronic eosinophilic pneumonia, acute eosinophilic pneumonia has an abrupt onset that progresses rapidly to respiratory failure. The first series of cases was described by Allen and colleagues in 1989, although earlier cases likely simply went unrecognized for many years. A young college exchange student treated at Tufts New England Medical Center presented to the emergency department with 48 hours of malaise, cough, and shortness of breath. She was febrile and looked acutely ill. Her peripheral blood did not manifest an eosinophilia, and her chest X-ray revealed a diffuse, somewhat nodular interstitial and alveolar process. Within 48 hours she was in the intensive care unit with hypoxemic respiratory failure (Figure).
Acute eosinophilic pneumonia has the following features: an acute febrile illness less than 5 days in duration; acute hypoxemic respiratory failure on presentation or within hours of presentation; diffuse mixed alveolar and interstitial infiltrates on chest X-ray. Diagnosis is established by demonstrating eosinophilia on BAL. Typically in acute eosinophilic pneumonia, the peripheral blood eosinophil count is normal. Bronchoscopic lavage also serves to rule out parasitic and other infectious causes. The response to systemic steroids is prompt and dramatic, and interestingly, recurrences are rare. A 2-4 week course of treatment usually suffices to achieve complete recovery to normal.
Most of the reported cases of acute eosinophilic pneumonia come from Japan. They have describe mostly adult patients, although cases have been reported as young as age 13. Most patients do not have pre-existing lung disease, including asthma. There is a possible association, however, with the recent initiation of cigarette smoking.
Lung pathology shows shows eosinophils and edema in the alveleor spaces and bronchial walls with no vasculitis. Small eosinophilic pleural effusions may accompany the pulmonary pathology.
The differential diagnosis of acute eosinophilic pneumonia includes infectious and toxic etiologies of diffuse acute lung injury. In particular, a large inhalational innoculum with fungi such as coccidiodes or aspergillus can cause an acute, diffuse pneumonia with pulmonary eosinophilia. Hyperinfection with strongyloides will rarely cause disease of this abruptness and severity. Finally, illicit drug use, particularly with crack cocaine, can cause the same clinical picture.
References:
1. Liebow AA, Carrington CB. The eosinophilic pneumonias. Medicine 1969; 48:251-85
2. Carrington CB, Addington WW, Goff AM, et al. Chronic eosinophilic pneumonia. N Engl J Med 1969; 280:787-98.
3. Boyce JA. The pathobiology of eosinophilic inflammation. Allergy Asthma Proc 1997; 18:293-300.
About the Author:
Dr. Joshua A. Boyce is an allergist and pediatric pulmonologist with appointments at the Massachusetts General Hospital and Brigham and Women’s Hospital. He directs the Partners Asthma Center at the MassGeneral Hospital for Children.