Partners Asthma Center Grand Rounds
Joshua A. Boyce, M.D.
The Spectrum of Eosinophilic Pneumonias Part 2
Eosinophilic Pneumonias with Other Systemic or Pulmonary Disease
Churg-Strauss Syndrome
Churg-Strauss syndrome is a systemic vasculitis that causes pulmonary eosinophilia, along with eosinophilic involvement of multiple other organs. It occurs predominantly in persons with preexisting asthma and allergic rhinitis, and it tends to be a more severe disease among those whose asthma is of recent onset. Peripheral blood eosinophilia is uniform and often quite striking, with eosinophil counts on the order of 1000-2000 cells/mm3 or greater. A prodromal period of marked peripheral eosinophilia may precede organ involvement.
Churg-Strauss syndrome uniformly involves the lungs. Infiltrates may be patchy and transient or have a more dense, alveolar-filling pattern. Small, non-cavitating nodules are common (and distinguishable from the larger, often cavitating nodules of Wegener’s granulomatosis). The diagnostic pathologic features are necrotizing extra-vascular granulomas combined with eosinophilic inflammation involving the vessel walls, giving rise to the other name for the disease: allergic angiitis with granulomatosis. Eosinophilic infiltration of alveolar walls and alveolar spaces may resemble the findings in chronic eosinophilic pneumonia, with which one finds some overlap.
Generally, the disease affects persons in early middle age, men and women equally often. Recent reports have identified more than 100 cases of Churg-Strauss syndrome among asthmatic adults recently begun on treatment with a leukotriene receptor antagonist. The possible causal relationship between Churg-Strauss syndrome and leukotriene blockers remains debated.
Potential extrapulmonary manifestations of Churg-Strauss syndrome are listed in Table 1. Skin involvement takes the form of cutaneous nodules, palpable purpura, or, in some instances, urticaria. The peripheral nervous system can be affected, manifesting a mononeuritis multiplex. Gastrointestinal symptoms may be prominent, including abdominal pain, diarrhea, and gastrointestinal bleeding as manifestations of an eosinophilic gastroenteritis. Heart failure is the most common sign of cardiac involvement. As in the idiopathic hypereosinophilic syndrome, one typically finds an eosinophilic myocarditis early on, followed by endomyocardial fibroelastosis. Renal lesions are varied in histology, but in general they respond more favorably to therapy than the glomerulonephropathies of Wegener’s granulomatosis or polyarteritis nodosa.
Distinctive laboratory features suggesting vasculitis as the cause of pulmonary eosinophilia include a high sedimentation rate, anemia, and in approximately 50%, a positive antinuclear cytoplasmic antibody (ANCA) test (p-ANCA or myeloperoxidase antibody). The IgE level is often high and may track with disease activity, decreasing and even returning to normal as the disease remits.
Aggressive treatment is warranted because of the systemic and potentially fatal nature of the disease. High-dose systemic corticosteroids are the mainstay of treatment. Many clinicians would add immunosuppressive agents such as cyclophosphamide, particularly in the patient with renal involvement. Fortunately, compared with Wegener’s granulomatosis or polyarteritis nodosa, prognosis is generally good.
Allergic bronchopulmonary aspergillosis (ABPA)
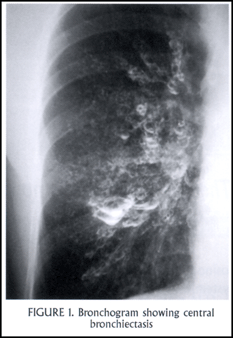
The first series of patients with ABPA was reported in the early 1950s. More than an eosinophilic pneumonia, this disease is primarily an intense eosinophilic response in the airways to aspergillus (and occasionally to other fungi as well). Aspergillus colonization of the lower respiratory tract occurs in people with abnormal mucus, specifically in people with pre-existing asthma or cystic fibrosis. An abnormal, exaggerated response to aspergillus antigens leads to damage of airway walls, causing a characteristic finding of central bronchiectasis in most cases [Figure 1: CT with central bronchiectasis].
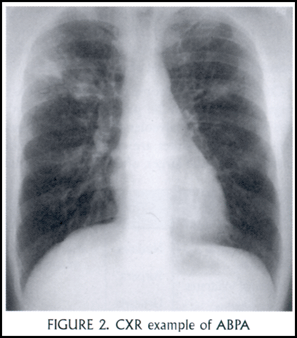
Mucus and inflammatory debris filling dilated central airways gives the finding of mucoid impaction. Radiographically, dilated, mucus-filled bronchi create what is described as a “finger-in-glove” appearance [Figure 2: CXR example]. Upper lobe involvement is most common, and there may be atelectasis or consolidation distally. Pathologically, one finds an inflammatory infiltrate in the large bronchi consisting of eosinophils, lymphocytes, plasma cells and monocytes, as well as the hyphae of aspergillus. Expectorated mucous plugs – taking the form of bronchial casts – when examined microscopically reveal features pathognomonic for chronic obstruction of the bronchi with eosinophils: Charcot-Leydon crystals (extracellular crystals consisting largely of eosinophil-derived lysophospholipase), Curschman’s spirals, and sheets of eosinophils. It is worth noting that other fungi may elicit an identical reaction, referred to generally as an allergic bronchopulmonary mycosis. These include candida, Helminthosporium, and Curvularia.
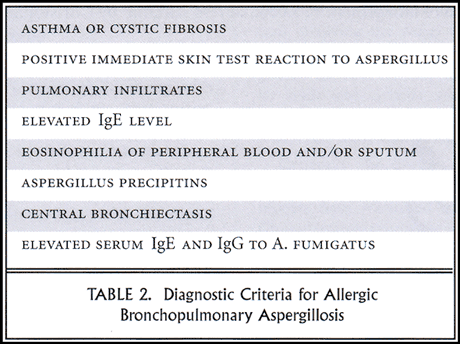
Although we are discussing it today under the category of eosinophilic pneumonias, ABPA is primarily a bronchocentric disease. Pulmonary eosinophilia, as evidenced by migratory pulmonary infiltrates, occurs, but it is a less prominent feature of the disease. A list of the 8 diagnostic criteria for ABPA follows (Table 2).
The therapy of ABPA is evolving. Traditional treatment has been with systemic steroids only, directed at the abnormal inflammatory response to the fungal colonizer. More recently, evidence from a randomized, double-blinded, placebo-controlled trial of itraconazole for ABPA clearly shows that anti-fungal therapy can be efficacious in reducing the steroid burden in these patients. It is likely that voriconazole, with its greater activity against aspergillus, will play an important role in treating ABPA in the future.
Tropical eosinophilia
Tropical eosinophilia is a disease of the third world. In southeast Asia, China, Africa, and especially in India, it is a common problem and can often be diagnosed based on its clinical presentation alone. In the United States, cases are exceedingly rare, most cases presenting after transcontinental travel.
Tropical eosinophilia is a sub-acute, systemic disease caused by an exaggerated host response to filarial parasites (W. bancrofti, B. malayi). Filaria gain access via mosquito bites and cause obstructive lymphatic disease. In children, the lymphatic involvement may be dramatic. Children can become very ill, and their presentation — with lymphadenopathy and hepatosplenomegaly — can mimic an acute leukemia with eosinophilia. The pulmonary manifestations are usually paroxysmal, nocturnal cough and hemoptysis. Hemoptysis results from direct invasion of the pulmonary capillaries by the migratory larval form of the filarial parasites. Over a period of a few weeks, wheeze, dyspnea, fever, and weight loss develop. For some reason, men are affected more often than women, by a ratio of 4:1
Peripheral blood eosinophil counts are uniformly high in tropical eosinophilia. Serum IgE levels also tend to be markedly elevated. The chest X-ray may be normal, but more typically it shows a diffuse, reticulonodular pattern with basilar prominence. Hilar lymphadenopathy may be seen, especially in children.
Pulmonary pathology in tropical eosinophilia evolves through several stages. It begins as a histiocytic alveolar infiltrate followed by massive eosinophilic infiltration of alveolar spaces and interstitium. Patients may go on to develop eosinophilic abscesses. Microfilariae may be found in the lung, liver, and lymph nodes.
The treatment of choice is diethylcarbamazine. Unfortunately, with initiation of therapy with diethylcarbamazine, patients can acutely worsen. A large amount of filarial antigen is released when the worms die, eliciting an acute immunologic reaction. Many clinicians have advocated using corticosteroids in conjunction with diethylcarbamazine. Use of systemic corticosteroids also tends to hasten resolution of the pulmonary disease, as is typical for eosinophilic pneumonias..
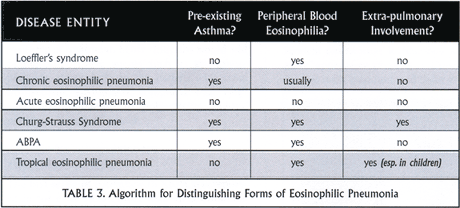
Assessment of eosinophilic pneumonias: A modern algorithm
Asking three specific clinical questions helps you to sort through the causes of eosinophilic pneumonia. These are:
- Does the patient have pre-existing asthma?
- Is there peripheral blood eosinophilia? and
- Is there extra-pulmonary involvement?
Table 3 demonstrates how answers to these questions can narrow your differential diagnosis.
General comments about treatment
At the present time, the treatment recommendations for eosinophilic pneumonias are relatively easy: steroids, steroids, and more steroids. Steroids are effective anti-eosinophil agents via a number of mechanisms. In a variety of cell lines, they suppress the expression of cytokine genes, including IL-5 and Gm-CSF, genes that contain a glucocorticoid-binding element. Steroids also directly act on eosinophils by enhancing their entry into programmed cell death (apoptosis). Apoptotic eosinophils do not cause tissue damage because they are rapidly engulfed and cleared phagocytically. Eosinophilia typically clears very rapidly, often after the first dose or two of prednisone at 1 mg/kg or 60 mg per day.
As a reminder, be wary of the patient who lives in or has traveled to areas endemic for strongyloides. Walking barefoot in contaminated dirt is a risk factor for infection with strongyloides; endemic areas include Southeastern United States and tropical climates further south. Steroid treatment can worsen disseminated strongyloidiasis.
In refractory cases of eosinophilic pneumonia or in cases where a steroid-sparing agent is sought, options include interferon alpha and immunosuppressive agents such as cyclophosphamide and azathioprine. Interferon alpha was originally introduced to treat resistant cases of idiopathic hypereosinophilic syndrome. There are a few case reports describing successful management of chronic eosinophilic pneumonia with alpha interferon as a means of reducing the dose of oral corticosteroids. Of course, interferon alpha has its own untoward side effects, including fever, joint aching, and malaise. Cyclophosphamide and azathioprine have been most useful as adjunctive therapy in cases of Churg-Strauss vasculitis.
It is likely that in the next 3-4 years we will see several new drugs introduced with potential application for treating the eosinophilic pneumonias. A humanized monoclonal anti-IL5 antibody is currently in clinical trial for asthma. Whether or not it proves useful for asthma, it will likely have a niche market in managing diseases characterized by eosinophilic tissue damage, such as acute and chronic eosinophilic pneumonias. In addition, small molecule inhibitors of the adhesion molecule, VLA-4, are undergoing clinical trial; and several pharmaceutical companies are eyeing chemokine receptor 3 (CCR3) as a potential target for the development of low molecular weight inhibitors.
It is likely that novel therapeutic approaches to eosinophilic lung diseases will emerge in the near future. Not only will these therapies will be exciting for treatment of these rare diseases, they will also have far reaching implications for the treatment of more common diseases that we treat everyday, such as asthma.
References:
1. Stevens DA, Schwartz HJ, Lee JY, et al. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med 2000; 342: 756-62.
2. Lilly CM, Churg A, Lazarovich M, et al. Asthma therapies and Churg-Strauss syndrome. J Allergy Clin Immunol 2002; 109:S1-19.
3. Ong RK, Doyle RL. Tropical pulmonary eosinophilia.
Chest 1998; 113:1673-9.
About the Author:
Dr. Joshua A. Boyce is an allergist and pediatric
pulmonologist with appointments at the Massachusetts General
Hospital and Brigham and Women’s Hospital. He directs the
Partners Asthma Center at the MassGeneral Hospital for Children
as well as a research laboratory focused on eosinophil and mast
cell development.
Return to Text

Return to Text