Partners Asthma Center Grand Rounds
David C. Christiani, M.D.
Update on Occupational Asthma
A 32-year-old auto-body worker with a productive cough for several years presented with a one-year history of worsening cough and shortness of breath at work. He was often awakened at night with symptoms, especially during the work week, and he had begun to develop some chest tightness. Early in his course, his symptoms were clearly worse almost immediately when he began work on Mondays, and they improved significantly on weekends. Although he now had symptoms throughout the entire week, he experienced noticeable improvement when he was away from work for several days. He worked for eleven years in auto-body repair, where he did a lot of spray painting. He often used urethane paints containing isocyanates. Although now a manager, he still supervised staff who did spray painting. He had begun using a face-mask respirator with an air purifier cartridge.
He had no prior history of asthma or allergies. He had quit smoking 10 years ago.
On initial evaluation (after a work shift on a Tuesday), he had a few end-expiratory wheezes on chest exam. Spirometry revealed an FEV1 79% of predicted with significant improvement following bronchodilator. The following week, pre- and post-shift spirometry on a Monday demonstrated a 20% drop in his FEV1.
Definitions
Occupational asthma can be defined as disease characterized by asthma due to causes and/or conditions attributed to a particular occupational environment. We have to differentiate occupational asthma from pre-existing asthma aggravated by workplace exposures. Established asthma made worse by nonspecific irritant stimuli in the workplace is probably more common than true occupational asthma, and is referred to as occupationally-aggravated asthma.
One can distinguish two types of occupational asthma. The most common is asthma with a latency period. This is a group of asthma syndromes due to over 250 compounds that have been well established to cause asthma after a period of sensitization. It encompasses all of the cases of immunologic asthma for which a mechanism has been identified or postulated. It includes most of the high- and some of the low-molecular weight compounds about which we will speak.
Asthma without a latency period has also been called irritant-induced asthma or "reactive airways dysfunction syndrome." It typically occurs after high-dose exposure to compounds that are generally not considered sensitizing, such as irritant gases, smoke, and other compounds. Examples include ammonia, chlorine, and hydrochloric acid, compounds that are not sensitizers or allergens.
Some chemicals, such as the isocyanates, are active immunologically as sensitizers and can also act as irritants at high doses. A terrible example resulted from an explosion at a plant that made methyldiisocyanate (MDI) in Bhopal, India. Although MDI is a powerful sensitizer, most of the injuries were the acute consequence of high-level exposure, including blindness, chemical bronchitis, chemical pneumonitis, and asthma without a latency period.
Prevalence
Among the general adult population, it has been estimated that 2-5% of patients with asthma have occupational asthma. Among populations at risk because of their exposure to known sensitizing agents, the risk of developing occupational asthma can be as high as 5-10% per year.
In fact, defining the precise prevalence or incidence of occupational asthma is difficult. Cases are often overlooked, by generalists and specialists alike. Another problem is referred to as "healthy worker selection bias." Persons may enter a workplace, become sensitized to a compound in the work environment, and then voluntarily leave the job, perhaps during an initial probationary period. The prevalence of occupational asthma then refers to the prevalence in the survivor population, a so-called healthy worker selection bias.
The relatively small number of cases distributed across many businesses makes occupational asthma hard to study. Resources needed to confirm the diagnosis and to conduct proper industrial hygiene evaluations are often lacking. In addition, physician compliance with state laws mandating case reporting (SENSOR program sponsored by the CDC) is often poor. In Massachusetts only approximately 46 cases of occupational asthma were reported last year, in contrast to approximately 4000 cases reported in California, where compliance with reporting has been very good. This difference cannot be fully explained by differences in population or density of the manufacturing sector between the two states.
Workers themselves often underreport their work-related symptoms for fear of job loss. One often hears of malingering workers seeking compensation for imaginary symptoms, but the converse is also true: workers who work sick. My patient, presented as the case above, said, "There is no way I can get another job with just a high school education that pays me $60,000 a year." The work-related disability can be rather insidious. Sometimes workers can adapt to their work environment by having other workers do some of their tasks, allowing them to stay away from those materials that bother them.
Risk Factors
Three factors influence the risk of developing occupational asthma: 1) the nature of the compounds themselves and their inherent toxicity; 2) the level of exposure to them; and 3) host factors, both genetic and behavioral (e.g., cigarette smoking). We will deal with each one briefly.
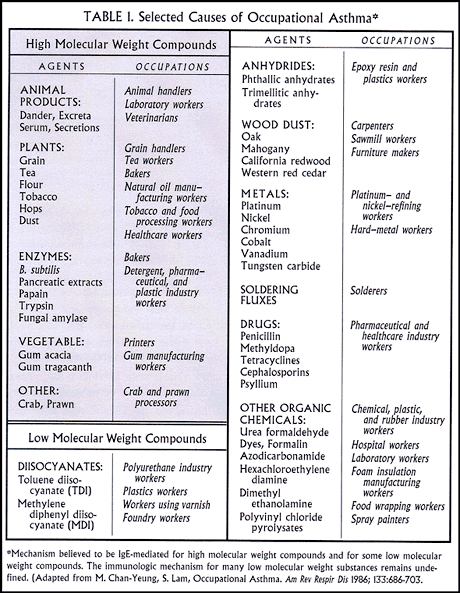
The sensitizing compounds, often referred to as asthmagens, fall into two broad categories according to their molecular weight. The high molecular weight group (>1000 daltons) includes proteins (like rubber proteins and latex), polysaccarides, and peptides. In most instances these compounds elicit the classic IgE antibody response and typical immediate and/or delayed asthmatic reactions. Low molecular weight compounds are mostly modern industrial chemicals rather than animal- and plant-derived compounds. Examples include the isocyanates and hydrides, epoxies, antibiotics, formaldehyde, and glutaraldehyde. Some of these compounds are definitely haptenic. They combine with carrier proteins and induce an immunologic response, more often an IgG than IgE antibody response. However, In many instances the immunologic mechanism remains uncertain.
One can never remember all of the compounds that are asthmagens. The best approach is to keep families of agents or groups of work situations in your mind (Table 1). If you encounter a patient whom you think might have this type of exposure, you can then just look it up in the appropriate textbook.
The level of exposure to the asthmagen is an independent risk factor for developing occupational asthma. It appears that for many compounds, short episodes of high-intensity exposure are as sensitizing as longer periods (months or years) of low-level exposure. This circumstance increases the difficulty of ensuring compliance with environmental standards in the workplace. A worker may become sensitized to a short burst of exposure in a workplace that is the rest of the time in compliance with air quality standards. Another confounding factor results from the positive feedback loop by which sensitized individuals will manifest asthmatic responses to lower and lower levels of the same material. Thus, levels of toluene diisocyanate considered below the permissible limit of exposure (<0.02 parts per million) may elicit a clinical response in an already sensitized worker.
Because sensitization can occur relatively rapidly, over a period of weeks to months, occupational asthma can cause loss of employment among young workers, many just out of school or job training. The economic and social implications of their job losses are enormous.
Among host factors predisposing to occupational asthma, atopy increases one's risk of sensitization to high-molecular weight compounds (plant and animal-derived materials) but not to low-molecular weight compounds. Consequently, It is probably legitimate to advise someone with cat allergies not to work in a veterinary office or animal laboratory, but not to discourage employment in electonics manufacturing, where there may be exposure to anhydrides, epoxies, or isocyanates. Cigarette smoking may potentiate development of asthma on exposure to a few sensitizing agents, particularly anhydrides and detergent enzymes, but for the most part it has not been shown to increase one's risk for developing occupational asthma. Likewise, heightened bronchial responsiveness (such as might be tested by pre-employment bronchoprovocation challenge) does not appear to be an independent risk factor. Polymorphisms at certain genetic histocompatibility locus antigenic types may prove to be important host factors, but we have only limited information at present. For the time being, the only clinically justified screening is taking a history for atopy and for any prior reactions to compounds that may have some homology to what the worker is going to be handling in the workplace.
Diagnosis
The history is critically important. Symptoms may include episodic wheezing, shortness of breath, and chest tightness, or any of these symptoms in isolation. Cough and sputum production in a nonsmoker should raise some suspicions. Upper airway symptoms such as rhinitis, experienced cyclically with the work week, may be a useful tip-off to sensitizing exposures. One's suspicion of occupational asthma should also be raised if other workers in the same environment have been diagnosed with asthma. Patients generally know what asthma is and what inhalers look like. If many co-workers are pumping on their inhalers in the lunch room, there may be a problem with the environment at the workplace.
Periodicity of symptoms, with symptoms worse at work or during the nights after work, is typical of occupational asthma. Some sensitizers, especially the low-molecular weight compounds, can elicit an isolated late asthmatic reaction. Symptoms may develop only in the evening hours after the exposure has stopped and may be erroneously attributed to allergens in the home environment.
Allergy skin tests and serologic tests (RAST) are of limited use in occupational asthma. There are no good skin or serologic tests available for most of the potential offending compounds, and even in those instances where one exists (e.g., RAST for latex allergy), a positive result indicates only sensitization and not occupational asthma.
Pulmonary function tests and methacholine bronchoprovocation challenge are useful to establish the presence or absence of asthma. To link asthma to occupational exposures, pre- and post-shift spirometry is helpful. A 15% fall in the FEV1 following work exposure is suggestive of the diagnosis. A negative result does not exclude occupational asthma, because of day to day variation in the workplace environment.
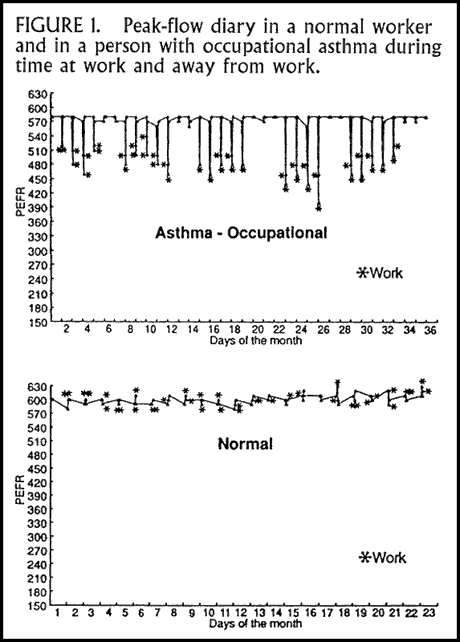
We make frequent use of peak flow measurements made repeatedly throughout the day and recorded in a diary over many days. Ideally, one uses the "stop-resume" method, during which careful documentation of peak flows is made during 10 days off work followed by 10 days back at work. An example of such a recording is shown in Figure 1. Normal diurnal variation of peak flow is less than 20%. The test is totally effort dependent, and patient compliance with the proper procedure is critical.
Although perhaps the most definitive test, bronchoprovocative
challenge with the potentially offending asthmagen is rarely
performed. Difficulties include the facts that we often are not
certain what agent is responsible (and it may not be a single
agent), what dose to give, how fast to give incremental doses,
and what the observation period should be. The risk of adverse
reactions, including delayed responses, makes this form of
testing unwise outside of specialized research settings.
Prognosis
The news here is disappointing: as many as 50% of persons with occupational asthma will retain their symptoms and/or bronchial hyperresponsiveness even years after withdrawal from the workplace.
Prognostic indicators favoring recovery after cessation of exposure include short duration of symptoms prior to diagnosis and withdrawal; less severe abnormality of lung function at time of diagnosis; and, in some studies, milder degree of bronchial hyperresponsiveness. Not predictive of recovery are age, race, cigarette smoking status, atopy, and the type of allergic reaction, whether immediate, late, or dual. One disconcerting study from England followed workers who insisted on remaining at work despite their development of occupational asthma. Even with their use of respirators and attempts to control exposures, the group as a whole had a further drop in FEV1 and increase in bronchial hyperresponsiveness over the next few years. No one got better.
Management
Once a diagnosis of occupational asthma has been established, even if one can't identify the specific agent or process causing the disease, one has only two choices: etiher remove the offending compound or process or have the patient leave the workplace. A job with the same employer in a different work area free of the exposure is best. This change may involve temporary disability and a retraining program. The patient has some rights and the employer some responsibilities in this regard under the Americans with Disabilities Act. However, loopholes in the act exist such that if a suitable job does not exist, the employer is not obligated to create one. If efforts are made to reduce or eliminate exposures in the work setting, careful follow up of the patient is needed to ensure clinical improvement.
Medical treatment of occupational asthma is the same as for any other kind of asthma, with institution of antiinflammatory therapies and bronchodilators. Patients who remain ill and unable to get another job two years after cessation of exposure need to be evaluated for permanent disability.
Occupational Asthma without Latency
A 29-year-old college student worked summers for a commercial cleaning company. One day while cleaning a floor, he and a coworker mixed two solutions, one containing ammonia, the other a chlorine-containing bleach, into large buckets. A low-hanging vapor cloud formed, and he began to cough right away. He experienced burning in his eyes, nose, and throat. His supervisor asked him to clean the area before evacuating it. He did this without any respiratory protection for about 15 minutes. His cough and chest tightness persisted after he ended the shift.
I saw him several weeks later. He was a young man in very good shape with an otherwise negative medical history. His exam was normal except for an occasional end-expiratory wheeze. Spirometry revealed an FEV1 that was 89% of predicted with cough during the spirometry. He had a positive methacholine challenge test with a moderate degree of bronchial hyperresponsiveness.
At three months he continued to have a cough, chest tightness on exertion, and cold air-induced bronchospasm. He began treatment with inhaled steroids and p.r.n. inhaled beta agonists. He was no longer working, but even at one year was still symptomatic.
Limited data are available about this condition. The original description identified persons with no history of atopy or bronchitis who developed asthma after a single massive exposure. There are some pathologic differences in bronchial biopsies from persons with irritant-induced asthma that distinguish it from sensitizer-induced asthma: more fibrosis in the bronchial walls, fewer T lymphocytes, and fewer eosinophils, features suggesting a nonimmunologic mechanism. In fact, we can think of occupational asthma without latency as more of an airway injury than as a disease. It is characterized by acute injury and followed by recovery, probably with reinnervation of the airways during recovery and an altered threshold of responsiveness during this reinnervation. Changing from a disease model to an injury model helps to make sense of how symptoms like these can develop in the absence of any background of bronchial hyperresponsiveness.
References:
1. Christiani DC and Wegman DH. Occupational Respiratory Disorder. In: Levy B and Wegman DH (eds), Occupational Health: Recognizing and Preventing Work-Related Disease, Third Edition, Little, Brown and Company, Boston, 1994.
2. Bernstein IL, Chan-Yeung M, Malo JL, Bernstein D. Asthma in the Workplace. Marcel-Dekker, New York, 1993.
3. Chan-Yeung M, Malo JC. Occupational Asthma. New Eng J Med 1995; 333:107-112.
About the author: Dr. David Christiani is Professor of
Medicine at Harvard Medical School and at the Harvard School of
Public Health and Director of Occupational Medicine at the
Massachusetts General Hospital.
Return to Text