Partners Asthma Center Grand Rounds
Jeffrey M. Drazen, M.D.
Leukotrienes and Asthma
When mast cells, eosinophils, and some lymphocytes and monocytes are activated, they can release a variety of chemicals, called primary effector molecules, that transduce the activating signal into the features that we recognize as asthma. Among these molecules are the leukotrienes, powerful agents for bronchoconstriction, microvascular leakage, mucus secretion, and eosinophil recruitment. In this, the first issue of the quarterly Asthma Grand Rounds Bulletin from Partners Asthma Center, we will review the biochemistry of the leukotrienes, their effects on airway function, evidence for their importance in asthma, and the newly available leukotriene-blocking medications that can interfere with their function and can be used to treat asthma.
Biochemistry of the leukotrienes
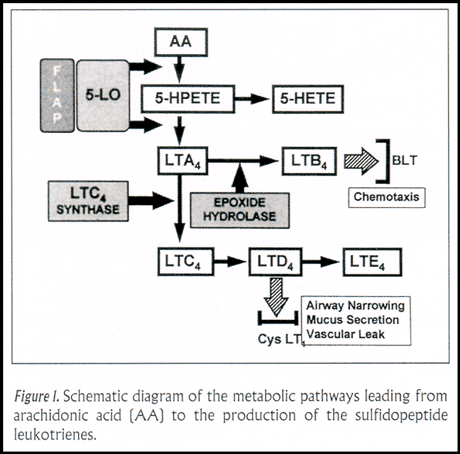
The leukotrienes are derived from membrane phospholipids. Two separate pathways leading to the formation of the leukotrienes have now been identified. One involves the enzyme cytosolic phospholipase A2 that acts on the perinuclear membrane to release arachidonic acid; the other involves secretory phospholipase A2, which clips off arachidonic acid from the cellular membrane separating the cytosol from the extracellular space. This latter pathway is equivalent to adding exogenous arachidonic acid that then diffuses into the cell to become a substrate for metabolism to the leukotrienes.
Arachidonic acid is metabolized to the leukotrienes by the action of the enzyme 5-lipoxygenase. A 5-lipoxygenase activating protein ("FLAP") is needed to interact with 5-lipoxygenase for generation of leukotrienes from arachidonic acid liberated from perinuclear membranes but not, apparently, for leukotriene formation from cellular membrane-derived arachidonic acid. Leukotriene A4 is an unstable intermediate derived from arachidonic acid. It becomes the substrate for LTC4 synthase, leading to the formation of leukotriene C4. A specific transmembrane LTC4 transporter then gets LTC4 into the extracellular space. LTC4 then is a substrate for gamma glutamyl transpeptidase, which cleaves an amino acid residue from LTC4 to form LTD4 (Figure1). LTD4 acts at the cysteinyl leukotriene receptor type 1, which when stimulated causes airway narrowing, mucus secretion, and vascular leak, actions that we recognize as the physiology of asthma. LTD4 can be acted upon by a number of dipeptidases to form LTE4. LTE4 also can stimulate the cysteinyl leukotriene receptor, but its potency is 100-fold less than LTD4.
Thus, for cells to make the primary effector molecules, leukotrienes C4, D4, and E4 (once known as slow-reacting substance of anaphylaxis and now known as the sulfidopeptide leukotrienes), they need enzymatic machinery that includes phospholipase A2, 5-lipoxygenase activating protein, 5-lipoxygenase, and LTC4 synthase. Mast cells and eosinophils, prominent in the airways of persons with asthma, have these enzymes.
Leukotriene Effects on Airway Function
Shortly after the structural identification of the leukotrienes in 1979, Sven-Erik Dahlen working in the Karolinski Institute in Sweden was able to take human bronchial strips that he isolated from the lungs of patients undergoing surgery for lung cancer and show that they would constrict to histamine. He then obtained a biosynthetic leukotriene C4 and showed that this substance was a thousand-fold more potent than histamine in terms of constricting the airways obtained in this way.
Work done here at the Brigham and Women's Hospital by Drs. Robert Lewis, Frank Austen, and me, in conjunction with the organic chemist at Harvard University, Dr. E. J. Cory, led to identification of the active stereoisomers of the leukotriene molecules. As a volunteer subject in one of our early experiments, I inhaled leukotriene D4 and histamine to compare their effects as airway constrictors. The concentration of histamine causing significant airway narrowing -- and I don't have asthma -- was approximately 30 millimolar. The concentration of leukotriene D4 producing the same degree of bronchoconstriction was approximately 3 micromolar or ten thousand times less. In addition, the duration of bronchoconstriction induced by the leukotriene was more prolonged, on the order of 30 minutes as opposed to approximately 10 minutes for histamine. Thus, we were able to demonstrate that the leukotrienes are among the most potent bronchoconstrictor substances known to man and have a sustained duration of action.
Recovery of leukotrienes from patients with asthma
Equally important in demonstrating the importance of leukotrienes in the pathogenesis of asthma has been the recovery of leukotrienes from patients with asthma. Leukotrienes are exceedingly difficult to measure and quantify in the serum, but leukotriene E4, the metabolic product of LTC4 and LTD4, can be recovered from the urine and serve as an indicator of leukotriene production. Dr. Grant Taylor and his group in England were the first to show that persons with allergic asthma inhaling an allergen to which they are sensitive will develop symptoms of asthma and a significant increase in the amount of leukotriene E4 recovered from their urine.
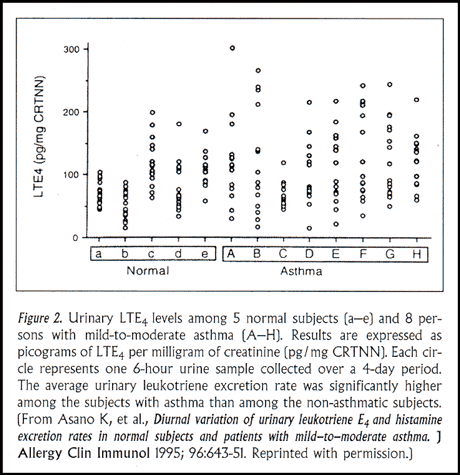
One of the critical points that these investigators demonstrated is that not everyone with severe asthma makes abnormally large amounts of leukotrienes. Although their asthma may appear indistinguishable in all other ways, some people will make large amounts of leukotrienes as part of their asthma and other people will not. We were able to confirm this fact in two distinct settings. In one study, patients with generally mild and stable asthma were admitted to our clinical research center for measurement of their 24-hour urinary LTE4 production (Figure 2). Of these eight research subjects, some had persistently elevated levels of leukotrienes in their urine, whereas others had urinary LTE4 levels that were indistinguishable from persons without asthma.
In another study we meaured the level of LTE4 in the urine of
approximately 100 patients presenting to our Emergency Department
for care of acute attacks of asthma. We found that in
approximately two thirds of the patients, the amount of LTE4 in
the urine was increased; in approximately one third of acutely
ill asthmatics there was only a low level of urinary LTE4.
Incidentally, patients with hyperproduction of leukotrienes (high
urinary LTE4 levels) were more likely to have airway obstruction
that was rapidly responsive to bronchodilator therapy.
Treatment of asthma with medications that block the action or production of leukotrienes
Proof of the importance of leukotrienes in the pathogenesis of asthma requires evidence that blocking the formation or action of the leukotrienes makes asthma better. Of the potential sites for interfering with leukotriene metabolism, from cytosolic phospholipase A2, to FLAP, to 5-lipoxygenase, to LTC4 synthase, to specific leukotriene receptor antagonists, two classes of leukotriene blocking drugs have become available for clinical use. These are the LTD4 receptor antagonists and the 5-lipoxygenase inhibitors. Pranlukast, montelukast, and zafirlukast are leukotriene receptor antagonists; only the latter (Accolate®) is currently available in the United States.
Two trials performed in patients with mild-to-moderate asthma compared the effect of zafirlukast with placebo. The first study was designed to focus on control of asthmatic symptoms. Patients 12 years of age or older with an initial one-second forced expiratory volume (FEV1) >55% of predicted were eligible to participate. During a two-week run-in phase of the study, patients kept a symptom diary, rating their symptoms each day from 0 (no asthma symptoms) to 3 (asthma symptoms that interfered with normal daily functioning). Patients needed a cumulative one-week symptom score of at least 8 to continue.
Approximately 750 subjects were recruited into this study and were randomized 2:1 to receive zafirlukast. The mean FEV1 for the population as a whole was 78% of normal, confirming mild asthma. In the zafirlukast-treated group, symptom scores decreased significantly more than with placebo. A different way of looking at these data was to do a Kaplan-Meyer survival analysis. Six percent of patients treated with placebo experienced an asthma exacerbation during the 13-week study; only half as many subjects in the zafirlukast-treated group suffered an asthma exacerbation.
A similarly designed, double-blind trial of zafirlukast versus placebo compared the frequency of asthma episodes and health care contacts between the two groups. There were approximately 150 patients randomly assigned to each group. The number of days per month without asthma episodes was approximately 10 in the zafirlukast-treated group vs. approximately 5 with placebo. Health care contacts were reduced from 40 per 100 patients per month to 18.5 per 100 patients per month with zafirlukast. Days lost from work or school decreased significantly with zafirlukast, from 7 to 3.9 days per month. Finally, beta agonist use for relief of asthmatic symptoms declined significantly in the zafirlukast group, at the same time that lung function improved by approximatley 7% compared to baseline (a significant difference compared to placebo).
Dr. Elliot Israel and I at the Partners Asthma Center participated in a 6-month, randomized, multicenter trial of the 5-lipoxygenase inhibitor, zileuton (Zyflo®), in patients with mild-to-moderate asthma. Prior to entry into the study, subjects were using only inhaled beta agonists; their mean FEV1 was approximately 60% of normal. Subjects were randomly assigned to one of three different doses of zileuton or to placebo. The high dose (600 mg four times daily) corresponds to the currently recommended dose of zileuton.
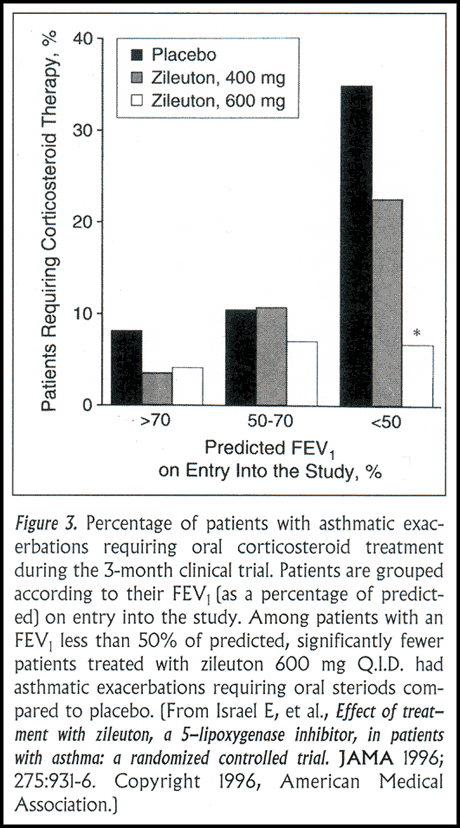
Two important outcomes in this study were the frequency of exacerbations of asthma requiring treatment with oral corticosteroids and the improvement in lung function as measured by the FEV1. Treatment with zileuton led to a significant reduction in the frequency of significant exacerbations, from 22% (placebo-treated group) to 8% (zileuton-treated group) (Figure 3). With respect to airways obstruction, the zileuton-treated group had clinically significant increases in their FEV1. When lung function was measured six hours after the last dose of zileuton, a trough drug effect, the mean increase in FEV1 was approximately 15-20% above baseline. When FEV1 was measured at peak drug effect, the mean increase was approximately 20-25%. Further analysis of these results reveals that not all patients derived benefit from treatment with zileuton. Approximately 40% achieved an increase in their FEV1 of 12% or more; approximately half of the group achieved an increase >10%. It is useful to observe the subgroup of patients who did not achieve an increase in their FEV1 of at least 5% after 8 days of treatment. Very few went on to have significant improvement over the ensuing weeks of treatment.
In a subsequent study to investigate the effect of zileuton dose reduction, patients were enrolled only if they had a 10% or greater increase in their FEV1 during a two-month, open-label, run-in phase of the study. Exactly half of the 278 patients experienced this degree of improvement. A remarkable observation from this study was that among this selected group of zileuton "responders," the average increase in FEV1 as 38%. In the phase of the study when the dose-schedule of zileuton was reduced first to 600 mg three times daily and then to 600 mg twice daily, much — although not all — of this improvement was sustained.
The observation that some but not all persons with asthma respond to the leukotriene-blocking drugs has made me suspect that there are biologic differences in the asthma of our patients, specifically in their endogenous production of the 5-lipoxygenase enzyme, that account for these patterns of response. In our laboratory we are currently engaged in experiments to characterize the 5-lipoxygenase gene and its various alleles. We have preliminary evidence to indicate genetic heterogeneity among persons with asthma in their ability to produce the 5-lipoxygenase enzyme; and I suspect that persons with high levels of 5-lipoxygenase production will be those most likely to respond well to this new class of medications, whereas persons with low levels of production will tend to be less responsive.
Figures