Partners Asthma Center Grand Rounds
Christopher H. Fanta, M.D.
Corticosteroids in the Management of Acute, Severe
Asthma
A 35 year-old woman with a long history of asthma presents to the emergency department (ED) five days after developing an upper respiratory tract illness. Her 3 year-old daughter and her husband had both been ill with colds during the previous week. She developed a sore throat, nasal congestion, and low-grade fever, and soon thereafter a productive cough, shortness of breath, and wheezing. She increased her inhaled steroid from 2 to 4 inhalations twice daily, but noted no improvement. She found herself needing to use her albuterol inhaler 4-5 times per day. After a sleepless night of cough and chest congestion, she sought help at her local hospital.
In the ED she appeared in moderate respiratory distress. She had labored breathing at 28 breaths/min, with a markedly prolonged expiratory phase. He was using her accessory muscles of respiration. Her blood pressure was 120/70 mm Hg with 20 mm Hg paradoxical pulse. Her heart rate was 112 beats/minute. Chest examination revealed musical inspiratory and expiratory wheezes throughout all lung fields.
Her peak expiratory flow rate (PEFR) was 150 L/min (33% of predicted).
You elect to administer corticosteroids to treat her airway inflammation: at what dose and by what route (inhaled, orally, or intravenously)?
Despite intensive treatment in the ED, she fails to improve sufficiently to be discharged home.
On hospital admission you write for administration of systemic corticosteroids: by what route and at what dose?
The following day she has improved only marginally.
Would you recommend doubling the dose of her systemic corticosteroids?
Over the next 2-3 days she progressively improves, and is now ready for discharge home.
At discharge, what recommendation do you have for her oral steroids: taper over one week, or over two weeks, or no taper at all?
In considering this case and the management of acute, severe asthma in general, we will address today the use of corticosteroids in the ED, upon discharge home from the ED, during hospitalization for continued treatment of a severe asthmatic exacerbation, and on hospital discharge. We will consider issues of route of administration, dose and duration of treatment, and the indications for the time-honored steroid “taper.”
In the emergency department
Severe airflow obstruction that develops acutely or subacutely yet fails to respond fully to intensive bronchodilator therapy is in most instances caused by airway inflammation. The treatment of choice for this predominantly eosinophilic bronchitis of asthma is corticosteroid therapy. In patients in whom there is persistent airways obstruction after the first few doses of bronchodilator, prompt initiation of steroids is recommended.
Nonetheless, the beneficial effect of corticosteroids given as part of ED therapy is not likely to be observed for several hours. The time course over which cellular infiltration, edema, and mucus hypersecretion are reversed is likely to be hours to days, as opposed to the minutes required for smooth muscle relaxation. As a result, in adults it has been difficult to demonstrate any impact from early initiation of systemic steroids on the outcome of care in the ED.
A study by Littenberg and Gluck published in the New England Journal of Medicine in 1986 suggested that administering methylprednisolone 125 mg as a single intravenous bolus at the start of ED therapy, along with traditional bronchodilators, would decrease the frequency of hospitalizations for asthmatic exacerbations compared to treatment with an intravenous placebo bolus. However, the differences in lung function at the time of disposition from the ED did not differ significantly between the two groups; and among the placebo group, 47% of patients required hospital admission, an atypically high frequency of admissions.
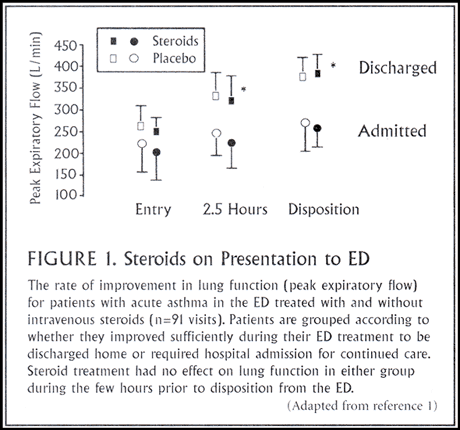
Subsequent studies, using a similar design, found no differences in lung function, length of ED stays, or frequency of hospitalizations among asthmatic patients treated immediately with intravenous methylprednisolone or placebo at the time of their presentation to the ED (Figure 1). Other investigators explored whether larger doses of steroids would be beneficial, but they could find no difference in outcome between patients treated with 125 mg or 500 mg of methylprednisolone.
Treating patients in the ED who have persistent severe airflow obstruction with corticosteroids remains the proper choice. Delay in administering the steroids will only prolong the time to recovery. Frequent bronchodilator treatments need to be continued at the same time, because the impact of steroid treatment will begin to be observed only after 6 or more hours – after the patient has been triaged out of the ED.
As we will discuss, orally administered steroids are likely to be equally effective as intravenous – in the absence of active vomiting. Orally administered steroids are fully absorbed with maximal blood levels achieved after one hour. The optimal dose of steroids in the ED is uncertain; most protocols call for between 60-120 mg of prednisone (or the equivalent), or 1-2 mg/kg in children.
Recent studies have explored the inhaled route of administration for corticosteroids in the ED, in the same way that we usually administer our bronchodilators by inhalation rather than systemically. One provocative study found that during the first 3 hours of ED care, patients given very high doses of inhaled flunisolide (Aerobid®) in combination with albuterol had more rapid recovery (as measured by FEV1) than patients given albuterol alone. The protocol called for 4 puffs of albuterol (400 µg) with or without 4 puffs of flunisolide (1000 µg) given every 10 minutes by metered-dose inhaler (MDI) with spacer for a total of 3 hours. Thus, the total steroid dose was 18 mg of inhaled flunisolide. It has been debated whether the rapid benefit observed might be due to a topical, vasoconstrictor effect of the inhaled steroid, reducing mucosal edema, rather than the usual anti-inflammatory effects associated with steroid use.
More recently, in a protocol conducted in asthmatic children old enough to perform spirometry, use of inhaled steroids was compared directly with oral steroids. In children, systemic steroids have been found to hasten improvement in asthma during ED care and to reduce the need for hospital admission. This difference in responsiveness compared to adults is unexplained. However, in this comparison of inhaled steroids (fluticasone 2 mg by metered-dose inhaler with spacer as a single dose) with oral steroids (prednisone 2 mg/kg as a single dose) among 100 asthmatic children aged 5 – 17 years, inhaled steroids proved inferior. Systemically administered steroids led to significantly greater improvement in lung function and fewer hospital admissions than did inhaled steroids.
On discharge from the emergency department
On average, 75% of patients can be discharged home after receiving treatment for asthmatic exacerbations in the ED. In all but a small minority of patients, discharge treatment should include systemic and inhaled corticosteroids. The frequency of relapses to the ED with recurrent asthmatic attacks and also the frequency of troublesome post-discharge symptoms of asthma fall significantly when systemic steroids are added to a program of bronchodilator therapy. Two protocols demonstrating this benefit used oral steroids: prednisone 40 mg in one, methylprednisolone 64 mg in the other, tapered to zero over 14 days.
In the patient in whom medication compliance is doubted, an alternative approach is the use of intramuscular corticosteroids. Repository steroids, using a special preparation of “depo-“ methylprednisolone or triamcinolone administered intramuscularly, has proved equally efficacious as a “short course” of oral steroids in preventing asthmatic exacerbations in the seven days following ED discharge. Two reported clinical trials used widely different doses: in one, 80 mg of methylprednisolone sodium succinate was administered; in the other, 240 mg of the same medication. The optimal dose has not been defined.
The anti-inflammatory effects of systemic steroids wane rapidly after they are stopped. If inhaled steroids are also begun at the time of ED discharge, asthmatic attacks diminish in frequency during the weeks and months after stopping systemic steroids. These observations come as no surprise. It has been well established that inhaled steroids decrease the frequency of asthmatic exacerbations, including severe exacerbations necessitating ED care and hospitalization. An asthmatic attack treated in the ED is an appropriate indication to begin regular controller therapy with an inhaled steroid.
During hospitalization
The role of systemic corticosteroids in hospitalized patients with acute, severe asthma was established in a randomized, double-blind, controlled clinical trial comparing the addition of intravenous hydrocortisone vs. placebo to very intensive bronchodilator therapy. The regimen of bronchodilators used at the time (1980) included intravenous aminophylline, inhaled beta-agonist (isoetharine) by nebulizer every 2 hours, and subcutaneous terbutaline every 6 hours. In the group treated with bronchodilators only, average improvement in lung function was slow and did not reach significance (compared to baseline) until 18 hours into the course of in-hospital treatment. Five of the nine patients randomized to receive only bronchodilators (plus placebo) deteriorated or improved by less than 10% at the end of the 24-hour study; all of the eleven patients in the steroid-treated group improved. For a patient weighing 70 kg, the dose of steroids administered was approximately 1000 mg of hydrocortisone over 24 hours, equivalent to 200 mg of methylprednisolone.
Systemic corticosteroids are widely accepted as the standard of care for hospitalized patients with severe asthma. However, despite this consensus on the use of systemic steroids, there remains ample debate regard the optimal route of administration and dose. Several studies have compared oral vs. intravenous administration of steroids during hospital-based treatment for asthma, finding no differences in outcome when comparable doses are administered by the two routes. That is to say, orally administered steroids have been shown to be equally effective as intravenous steroids for in-hospital treatment of acute, severe asthma. At the Brigham and Women’s Hospital, our inpatient asthma care path calls for orally administered prednisone 60 mg every 8 hours during the first hospital day, tapered to 60 mg twice daily on the second hospital day and 60 mg once daily on the third hospital day.
In an attempt to define the optimal dose, several studies have compared low vs. high doses of systemic steroids (administered orally in some studies, by intermittent intravenous bolus in others). The high doses ranged from 3 to 10 times greater than the low doses. With one exception, these studies found no differences in outcomes (rates of improvement in lung function or duration of hospitalization) between the lower and higher doses. All of these studies shared the common shortcoming of small sample sizes; the largest study recruited fewer than 70 patients. As a consequence, type II statistical errors might account for some of the negative results. On average, the low dose used in these studies was the equivalent of prednisone 50-100 mg/day (in divided doses).
Recommendations based on reviews of the published literature vary considerably. In a review published in 1993 in the American Review of Respiratory Diseases, Dr. Regis McFadden concluded that somewhere between 150-225 mg of prednisone or the equivalent would be optimal. The Expert Panel of the National Asthma Education and Prevention Program recommended 120-180 mg/day. On the other hand, the British Thoracic Society proposed as part of their National Asthma Guidelines that in-patients with acute, severe asthma be treated with the equivalent of prednisone 30-60 mg/day. A large-scale, likely multi-center trial will be needed to define more precisely a minimum effective steroid dose in this setting.
On hospital discharge
Hospitalized asthmatic patients treated with systemic steroids and intensive bronchodilator therapy will improve at variable rates. Although the average length of hospital stay for acute, severe asthma is on the order of 3-4 days, it takes as many as 7 days for 50% of patients to have their lung function return to normal (or to their individual baseline value) and as many as 10-14 days for 80% to recover fully. Said in another way, some asthmatic patients will respond quickly to treatment, others more slowly, some requiring as many as 2 weeks for full recovery. How long should systemic steroid treatment be continued?
A rational approach, it seems to me, is to continue systemic steroids in an individual patient until his or her lung function has returned to normal (or baseline), or close to it. Patients able to make accurate measurements using portable peak flow meters can dose their systemic steroids accordingly. When prescribing a steroid “course” at hospital discharge, it is reasonable to recommend continuing treatment with oral steroids until return to baseline of lung function, then discontinuing systemic steroids while continuing inhaled steroids. In the absence of reliable patient self-monitoring or close patient-physician communication, an alternative approach is to recommend 14 days of oral steroids. With this duration, the majority of patients will likely have experienced full recovery.
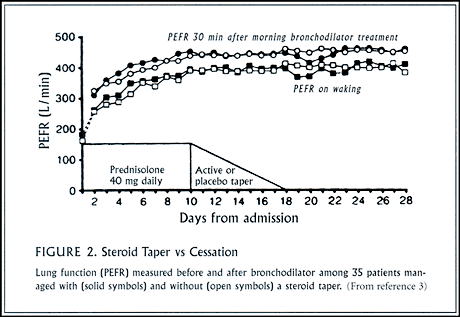
Although a steroid “taper” remains routine practice, its rationale is debatable. Abrupt cessation of systemic steroids will not provoke adrenal insufficiency when the duration of treatment is only 2-3 weeks. Recurrent, severe asthmatic attacks following abrupt cessation of systemic steroids are unlikely when patients continue on inhaled steroids. Potentially, a gradual dose reduction leads to fewer symptoms of steroid withdrawal and fewer overall adverse side effects compared to sudden discontinuation, but this advantage to the steroid taper has never been documented in a controlled trial, and it is equally easy to envision more adverse effects from the added length of use of steroids during a taper. Two British studies found no advantage to steroid taper following recovery of lung function to normal or near normal (>85% of normal or of baseline) following in-hospital treatment for acute, severe asthma (Figure 2). Once lung function has normalized, systemic steroids can be stopped. Gradually withdrawing oral steroids over 7-10 days had no benefit in terms of asthmatic symptoms, lung function, or recurrent exacerbations.
Conclusions
The following are my own conclusions from reviewing the published literature on the use of steroids in acute, severe asthma:
In Emergency Department,
- Give prednisone 60 mg to adult patients (1-2 mg/kg to children) who do not respond rapidly and fully to inhaled bronchodilators.
- At discharge, give a 7-day course of prednisone (beginning at 40-60 mg/day) together with inhaled corticosteroids to patients with less than maximal lung function.
- Intramuscular corticosteroids (e.g., methylprednisolone sodium succinate 80 mg) are an alternative in patients thought likely to be non-adherent with treatment.
- All patients should receive inhaled corticosteroids on ED discharge.
In hospitalized patients,
- Give prednisone 60 mg orally every 8 hours initially, tapering to 60 mg/day by day 3.
- Continue oral steroids until lung function returns to baseline or near baseline. One can then discontinue oral steroids without a taper.
- All patients should receive inhaled corticosteroids on hospital discharge.
References:
1. Stein LM, Cole RP. Early administration of corticosteroids in emergency room treatment of acute asthma. Ann Intern Med 1990; 122:822-7.
2. Fanta CH, Rossing TR, McFadden ER Jr. Glucocorticoids in acute asthma: a critical controlled trial. Am J Med 1983; 74:845-51.
3. O’Driscoll BR, Kalra S, Wilson M, et al. Double-blind trial of steroid tapering in acute asthma. Lancet 1993; 341:324-7.
About the Author:
Dr. Christopher H. Fanta is a member of the Pulmonary and Critical Care Division at the Brigham and Women’s Hospital. He is Director and co-founder of Partners Asthma Center.