Partners Asthma Center Grand Rounds
James A. MacLean, M.D.
Allergic Bronchopulmonary Aspergillosis
Case Example
A 23-year-old man was evaluated for asthma and bilateral pulmonary infiltrates. He had had asthma since childhood. Although he had occassionally received oral corticosteroids for asthmatic exacerbations as a child, he had never required hospitalization for asthma. Of note is that in the past two years he had been treated for pneumonia on two occasions. His current medications included inhaled corticosteroids and an oral antihistamine.
He was a lifelong non-smoker but had on occasion used marijuana.
On examination he looked well and had normal vital signs. Nasal and oropharyngeal exams were unremarkable, and he had no peripheral lymphadenopathy. Chest exam revealed expiratory wheezing bilaterally but no signs of consolidation. The rest of his examination was normal.
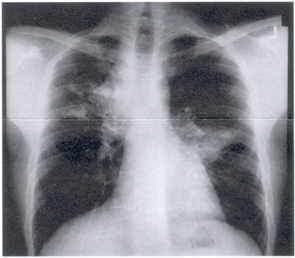
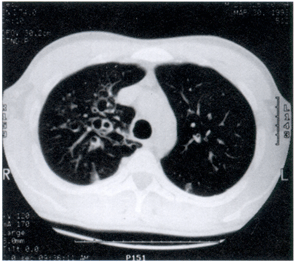
Laboratory studies of note included a normal total white blood cell count with 14% eosinophils on differential. His chest X-ray (Figure 1a) and chest CT scan (Figure 1b) revealed bilateral pulmonary infiltrates and central bronchiectasis.
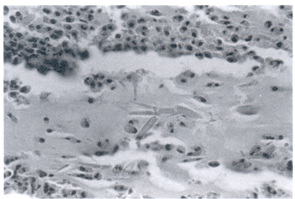
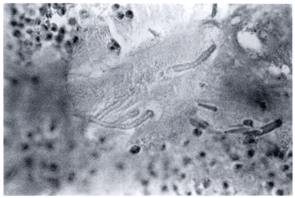
In this particular case bronchoscopy was performed, and a mass-like lesion was /identified endobronchially. The histopathology of this mucoid impaction revealed eosinophils, Charcot-Leyden crystals (Figure 2a), and acute-angle branching fungi characteristic of aspergillus (Figure 2b).
The Organism
More than 300 species of aspergillus have been identified, but by far the most important species in terms of causing human disease is Aspergillus fumigatus. The organism is ubiquitous and very heat-tolerant. It is typically found in decaying vegetable matter (and may contaminate marijuana joints). The size of the aspergillus spore, approximately 2-4 µ in diameter, makes it ideal for aerosolization and for inhalation into the lungs. All of us are repeatedly exposed to this organism, but normal host defense mechanisms, especially macrophages and polymorphonuclear leukocytes, protect us from disease.
Spectrum of aspergillus-related lung diseases Aspergillus can elicit a variety of different immunologic reactions, and, depending on the host, it can cause any of several different lung diseases, including allergic bronchopulmonary aspergillosis (ABPA). These entities are listed in Table 1.
Table 1:
|
||
Type of reaction |
Disease example(s) |
|
|
Immune reaction to exposure |
Asthma Hypersensitivity pneumonitis |
|
|
|
Underlying lung disease (e.g., COPD, bronchiectasis) Aspergilloma |
|
|
Airway colonization without invasion
but with intense immunologic reaction: |
Allergic bronchopulmonary aspergillosis |
|
|
|
Semi-invasive aspergillosis Invasive aspergillus pneumonia |
|
Pathology
In ABPA the dominant pathologic findings are eosinophilic pneumonia and bronchiectasis of the proximal bronchi ("central bronchiectasis"). Lung injury is caused not by direct invasion of the fungus but by the intense allergic reaction triggered by its colonization of the airways. Plugs composed of mucus, eosinophils, and cellular debris may fill dilated bronchi ("mucoid impaction"). Generally, the eosinophilic pneumonia and mucoid impaction are reversible with treatment; central bronchiectasis is irreversible. Occasionally, persistent eosinophilic pneumonia and associated atelectasis lead to scarring and irreversible fibrotic lung damage.
Epidemiology
ABPA can complicate two illnesses, asthma and cystic fibrosis. In cystic fibrosis, recent studies have suggested a prevalence of ABPA of approximately 6%. In asthma, the prevalence appears to be considerably less. One review of mold-sensitive asthmatic patients found 3-4% with clinical evidence for ABPA. Looking at the entire spectrum of persons with asthma, others have estimated a prevalence of ABPA closer to 1%. My own sense is that even this estimate is probably too high. Even at a large referral practice such as Partners Asthma Center, ABPA is found only rarely.
Clinical Presentation
Daily productive cough with purulent-appearing sputum, chronic dyspnea, fever, chest pain, and hemoptysis are classic symptoms of ABPA and when reported by a patient with asthma should lead one to suspect the diagnosis. However, many patients will have few symptoms or non-specific symptoms related to their underlying asthma, so that an abnormal chest radiograph may be the first indication of the presence of this complication. Other patients will be diagnosed in the process of evaluating severe, steroid-dependent asthma or recurrent pneumonias in an asthmatic patient.
Physical examination is generally non-specific. Focal inspiratory rales suggestive of a pneumonia or area of bronchiectasis may raise one's suspicion and lead to chest radiography.
Laboratory evaluation
Laboratory studies are required to confirm the diagnosis (Table 2). Peripheral blood eosinophilia is common. The serum IgE level is characteristically very high, generally greater than 1000 U/ml. Serum precipitins to aspergillus are generally positive in ABPA. However, as many as 10% of asthmatic patients without ABPA will also have positive aspergillus precipitins. Patients with ABPA will uniformly have positive immediate skin test reactivity to aspergillus antigen, but this finding is likewise non-specific and may be found in many mold-sensitive asthmatics without ABPA.
Table 2:
|
|
The most specific serologic tests for ABPA involve the measurement of IgE and IgG antibody specific for aspergillus. These aspergillus-specific antibodies are measured by enzyme-linked immunosorbent assay (ELISA) and are laboratory-dependent in their reliability. We have found that the laboratories of Dr. Paul Greenberger in Chicago and Dr. Ray Slavin in St. Louis provide the most reliable results.
Aspergillus can be isolated from the sputum in about 60% of persons with ABPA. Thus, a positive fungal culture of the sputum is helpful, whereas a negative culture does not rule out the diagnosis.
The chest X-ray in ABPA may reveal migratory pulmonary infiltrates, commonly located in the upper lobes and often associated with some component of volume loss (atelectasis), a reflection of the associated central bronchiectasis. Large mucus plugs within bronchiectatic airways (mucoid impaction) may manifest as ovoid masses, with their long axis following the orientation of central bronchi. The most sensitive test for detecting central bronchiectasis is chest CT scanning.
Diagnosis
Consider a diagnosis of ABPA in an asthmatic patient with any of the following: a typical clinical presentation as described above; unexplained pulmonary infiltrates; aspergillus isolated from the sputum; persistent high-grade peripheral blood eosinophilia; very high serum IgE level; or a positive aspergillus skin test.
On occasion you may be able to identify patients with all of the serologic evidence for ABPA (very high IgE level, aspergillus precipitins, aspergillus-specific IgE by RAST), along with peripheral eosinophilia, skin test reactivity to aspergillus, and aspergillus isolated from the sputum, but having a normal chest X-ray and chest CT scan. The absence of pulmonary infiltrates and central bronchiectasis makes the diagnosis of clinical ABPA difficult, raising the possibility that this patient simply has asthma with mold (aspergillus) sensitivity. Some physicians have suggested a separate category for ABPA based on serologic criteria alone (ABPA-S) vs. ABPA with evidence of central bronchiectasis (ABPA-CB). A two-fold or greater elevation of aspergillus-specific IgE and IgG titers, done in the proper laboratories, may discriminate ABPA-S from mold-sensitive asthma.
Staging
A proposed staging system for ABPA, developed by Drs. Paul Greenberger and Roy Patterson, is summarized in Table 3. It aids in categorizing patients and comparing groups of patients with similar clinical manifestations. This staging system does not imply an orderly progression from mild to severe in any individual patient.
Table 3:
|
|||||
| Stage |
Serum IgE
|
Aspergillus
Precipitins |
Peripheral
Eosinophilia |
Specific IgE
antibody |
Chest
X-ray |
| Acute | +++ | + | + | + | + |
| Remission | + | ± | - | ± | - |
| Exacerbation | +++ | + | + | + | + |
| Steroid dependent | ++ | ± | ± | ± | ± |
| Fibrotic | + | ± |
- |
± | + |
Two clinical points that emerge from consideration of this staging system are worthy of emphasis. One is that the course of ABPA is often characterized by recurrent exacerbations and remissions, the latter often requiring treatment with systemic corticosteroids. Exacerbations mimic the initial (acute) presentation, typically characterized by productive cough, fever, dyspnea, and pulmonary infiltrates, sometimes with chest pain or hemoptysis. The other is that among the subpopulation of asthmatic patients who require daily or alternate-day prednisone for control of their disease (steroid-dependent), the laboratory diagnosis of ABPA may be difficult. Repeated serologic measurements may be necessary, including some obtained when the prednisone dose has been tapered.
Treatment
Primary treatment for ABPA is with systemic corticosteroids to suppress the allergic response rather than with antifungal therapy targeted at the fungal organism. A typical steroid dose is prednisone 0.5 mg/kg for approximately 2 weeks or until the pulmonary infiltrates clear. We usually then taper the prednisone dose over approximately two months to an alternate-day regimen, then continue to reduce gradually the prednisone dose to zero. Close patient follow-up is crucial. We regularly monitor the total serum IgE, pulmonary function tests, and chest X-rays. Note that the serum IgE usually falls with treatment by 35-75% but commonly does not return to the normal range. Nonetheless, establishing a baseline value for serum IgE when the patient is in remission can be helpful, because a significant rise (defined as a doubling or more above the baseline value) is frequently associated with clinical exacerbations and the development of recurrent pulmonary infiltrates. Occasionally patients will have few or atypical symptoms, and the rising serum IgE level provides the best clue to a recurrence of disease activity.
Two newer forms of therapy are beginning to undergo clinical experimentation. One is the use of high-dose, high-potency inhaled corticosteroids. In the past inhaled antiinflammatory agents such as cromolyn and beclomethasone proved ineffective. However, with the recent availability of corticosteroid agents having increased topical potency, re-exploration of this approach to treatment is warranted.
Similarly, in the past, antifungal therapy with amphotericin B was viewed as too difficult and too toxic for chronic use. Furthermore, even with good tissue penetration of the antifungal agent, eradication of aspergillus superficially colonizing the airways proved difficult. Now with the availability of oral imidazole antifungal agents such as itraconazole, daily antifungal therapy can be administered chronically with low risk of toxicity. The efficacy of this approach is uncertain, although a small, preliminary investigation has suggested a possible adjunctive role for oral antifungal agents in ABPA.
Future directions
Among the evolving diagnostic and therapeutic strategies for ABPA are the following: identification and characterization of novel aspergillus antigens that would aid in the diagnosis of ABPA; the use of new antifungal therapies, ; and the development of animal models to study disease pathogenesis. A recent study of patients with ABPA suggests a possible role of abnormalities in the cystic fibrosis transmembrane regulator (CFTR) gene in disease pathogenesis.
Suggested readings:
Greenberger PA. Allergic bronchopulmonary aspergillosis. In: Middleton E, Reed C, Ellis E, et al., eds., Allergy, Principles and Practice. 4th ed. St. Louis; Mosby, 1993: 395-1414.
Greenberger PA, Patterson R. Application of enzyme-linked immunosorbent assay (ELISA) in diagnosis of allergic bronchopulmonary aspergillosis. J Lab Clin Med 1982; 99:288-93.
MacLean JA. The spectrum of pulmonary responses to aspergillus. In: Kradin RL, Robinson B, eds., Immunopathology of Lung Disease. Boston, Butterworth-Heinemann, 1996:288-99.
Patterson R, Greenberger PA, et al., Allergic bronchopulmonary aspergillosis: staging as an aid to management. Ann Intern Med 1982; 96:286-91.
About the Author
Dr. James A. MacLean is a member of the Partners Asthma Center at Massachusetts General Hospital, where he is an Assistant Professor of Medicine and member of the Allergy and Clinical Immunology Unit. His research interests including allergic bronchopulmonary aspergillosis and murine models of asthma.