Partners Asthma Center Grand Rounds
Anthony F. Massaro, M.D.
Exhaled Nitric Oxide as a Marker of Inflammation in
Asthma
An advertisement for a commercial nitric oxide (NO) analyzer poses the provocative question: “If airway inflammation is so important in asthma, why not measure it?” This question raises two additional questions, to be discussed in this presentation: what is the evidence that exhaled NO actually reflects airway inflammation; and does this information about airway inflammation have the potential to influence the care of asthmatic patients? Does it add anything to our history, physical examination, and traditional measurements such as peak flow or spirometry, which assess airway caliber rather than airway inflammation. The topics to be addressed in this presentation are: the molecule NO itself and its synthesis and molecular interactions; postulated effects that NO has in the lung; methods to determine NO concentrations in exhaled gas; evidence that exhaled NO reflects airway inflammation in asthma; and preliminary data about its potential for affecting patient care.
Nitric oxide and nitric oxide synthase
For a long time, NO was only considered as a toxic gas, a component of air pollution and acid rain. Beginning in 1980, investigators became interested in a chemical released from endothelial cells in vascular ring preparations that caused vasodilation, dubbed the “endothelial-derived relaxant factor.” In 1987, two independent research groups (led by Furchgott and Ignarro) identified NO as the molecule causing this smooth muscle relaxation. Since then there has been a virtual explosion of information about the many biologic roles of NO, including its functions in platelet aggregation, immune modulation, and neurotransmission. In 1992 NO was named “Molecule of the Year,” and since then its discoverers have been awarded the Nobel Prize.
NO is an interesting molecule for a number of different reasons. It is extraordinarily small (30 Daltons) and a free radical. It is highly reactive, causing it to have a very short half-life. It represents a new class of signaling molecules. These molecules are not stored and so their concentrations are entirely related to their rates of production and their reactions with other molecules. There are no molecular receptors for NO.
NO is formed by the reaction between the guanidinyl nitrogen of L-arginine, oxygen, and NADPH. This reaction is catalyzed by the enzyme nitric oxide synthase (NOS) and requires five cofactors. The end products are NO and citrulline. NOS is comprised of a variety of different isoenzymes. Originally classified based on the cell of origin (e.g., n-NOS for neuronal NOS and e-NOS for endothelial NOS), the classification has since been revised based on genotyping data and the finding of isoenzyme types in a variety of different cells. We now speak of Type I, Type II, and Type III NOS, and possibly a newly discovered mitochondrial NOS.
A different classification scheme reflects the function of the isoenzymes: some are constitutively expressed in cells (c-NOS) and some are induced in the presence of inflammation (i-NOS). In contrast to c-NOS, i-NOS (which is Type II NOS) is not calcium dependent; it is transcriptionally regulated; it is up-regulated by pro-inflammatory cytokines; and it produces significantly larger quantities of NO. Dr. Jeffrey Drazen and colleagues showed that in epithelial cells in vitro, NOS is up-regulated by the cytokines found in asthmatic airways, such as interleukin 1B, tumor necrosis factor alpha, and interferon gamma and down-regulated by corticosteroids. Specialized stains of airway tissue reveal that Type II NOS (i-NOS) is present in relatively large amounts in airway epithelial cells, as well as in inflammatory cells and neurons.
As mentioned, NO is a highly reactive molecule that can participate in a variety of metabolic reactions. It can be oxidized to nitrite and nitrate. It binds avidly to hemoglobin (more avidly than oxygen or carbon monoxide), forming met-hemoglobin. It can interact with thiols present in the airway, forming compounds called nitrosothiols. Nitrosothiols are very stable forms of NO equivalents that can donate NO to chemical reactions in the lungs. Finally, NO can react with the superoxide anion, leading to the production of peroxynitrite, a highly potent oxidant. Peroxynitrite has numerous inflammatory effects in the airway, including its role in contributing to sloughing of airway epithelial cells.
Postulated airway effects of NO
As you know, NO is a potent vasodilator in the lungs, used to treat pulmonary hypertension. In this presentation we are focusing primarily on its airway effects. In animal models, NO acts as a bronchodilator of mild to moderate potency. In humans, however, it is at best a weak bronchodilator. Its bronchodilator effect may be mediated through non-adrenergic, non-cholinergic neural pathways. In a variety of different organ systems, NO is the non-adrenergic, non-cholinergic neurotransmitter. In the airways, this pathway causes bronchodilation through an inhibitory function on airway smooth muscle. NO can also bind other free radicals, limiting their harmful airway effects. These homeostatic effects of NO would favor normalizing airway function in asthma.
On the other side of the equation, NO has direct and indirect pro-inflammatory effects in the airways. NO can influence the balance between helper-type T lymphocytes types 1 and 2, causing an increase in the TH2 cells found in allergic inflammation. It has direct cytotoxic effects and can act through cytotoxic intermediates, such as peroxynitrite. Its vasodilator role can promote edema formation from submucosal blood vessels. And it may have a significant chemoattractant role, recruiting eosinophils and neutrophils to amplify and perpetuate the inflammatory response (as shown in animal models). These mechanisms raise the possibility that NO may be not only a marker of airway inflammation in asthma but also part of its pathogenetic mechanism.
Measuring exhaled NO
The first reports of reproducible measurement of NO in exhaled gas appeared in 1991. A variety of methods are available to measure NO, but by far the most widely used technique is chemoluminescence. NO combines with ozone generated by the analyzing machine to form nitrogen dioxide and oxygen, and the reaction gives off light. The intensity of light emitted is proportional to the amount of nitric oxide. Two methods exist for collecting and determining exhaled NO in human breath using this chemoluminesence analysis, referred to as “off-line” and “on-line” methods.
The off-line method does not require the patient to be at the site of the NO analyzer. Exhaled gas is collected in an impermeable vessel, such as a Mylar balloon. There is no loss of NO in these balloons for up to 48 hours. In making these measurements, it is necessary to control for the concentration of NO in the ambient air. Typically, ambient air contains 50 parts per billion (ppb) or less of NO, although the Occupational Safety and Health Administration permits as safe rises of ambient NO into the parts per million range for short periods. The ambient NO concentration is often higher than the NO concentration in human airways. Thus, the equipment requires a scrubber to remove ambient NO as the patient inhales to total lung capacity before exhaling into the collection balloon.
The concentration of NO in exhaled gas is very sensitive to the flow rate during exhalation. This observation probably relates to the very short half-life of NO. NO is being continually produced along the airway walls and reaches equilibrium with the gas in the airway lumen as it passes by during exhalation. The slower the flow rate, the more time that is available for equilibration of NO and the greater the amount of NO recovered in the exhaled gas. In order to ensure a constant rate of flow, patients exhale through a fixed resistor, thereby maintaining a constant pressure and, consequently, constant flow. Using the off-line method, the result is reported as a single value for the mixed exhaled NO concentration.
On-line measurement is made with the patient at the NO analyzer. The patient again breathes in through a device that scrubs all ambient NO and exhales through a resistor to achieve a constant flow rate. Both the oral pressure and the concentration of exhaled NO are monitored continuously. There may be an initial peak of NO concentration at the beginning of exhalation, reflecting the sample of gas from the central airways (dead space); thereafter a steady state or plateau is achieved. With commercially available machines, the operator monitors the target pressure during exhalation; the instrument automatically selects the plateau value for exhaled NO concentration. For pediatric patients a computer program includes the visual task of trying to blow a balloon over water at a steady level. Advantages of this on-line method include the following: exclusion of any contamination from the central airways; elimination of potential error from faulty storage of the sample; instant feedback on how well the patient was able to perform the test; and use of lower flow rates. Additional influences on the resulting exhaled NO concentration may include: dietary factors (especially foods containing large amounts of arginine, such as spinach, turkey, certain seeds, and soy protein); breath-holding at the end of inspiration; repeated spirometry maneuvers; possible diurnal variations in NO production; and, in children, some variation with age.
Exhaled NO in relation to airway inflammation
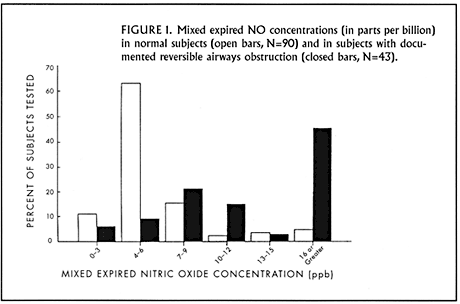
In an early study conducted here at the Brigham and Women’s Hospital, we compared the exhaled NO concentrations in 90 normal subjects with those found in 65 subjects who had a self-reported history of asthma. Using the off-line technique for NO measurement, we found the mean exhaled NO level in normal subjects to be 6.4 ppb, whereas in the asthmatic population it was 13.9 ppb. Separation between the two populations is shown in Figure 1. With further analysis of the asthmatic patients based on medication use, we found no differences in exhaled NO from beta-agonist or theophylline use, but a significant impact from the anti-inflammatory inhaled steroids. The subgroup not receiving inhaled steroids had exhaled NO values close to 20 ppb and significantly higher than those who were taking inhaled steroids.
In further exploration of this observation, we measured the levels of exhaled NO in 7 asthmatic patients at the time of their hospital admission for severe asthmatic exacerbations, then monitored their levels during the course of their treatment, which included systemic corticosteroids. In all 7 patients we found significant reductions in exhaled NO during treatment. The decrease in exhaled NO was concomitant with improvement in lung function and became statistically significant as early as 48 hours after beginning steroid therapy.
In a prospective analysis of the impact of anti-inflammatory therapy on exhaled NO levels, Dr. Peter Barnes’ group in England had 11 steroid-naïve asthmatic patients begin budesonide 1600 mcg daily. At the first follow-up assessment one week later, exhaled NO concentrations fell significantly. A corollary set of observations was made in Japan among a group of asthmatic patients dependent on inhaled steroids (at a dose equivalent to beclomethasone 1500 mcg/day or higher). After a two-week run-in period, their dose of inhaled steroids was reduced in half; and their mean exhaled nitric oxide concentration increased significantly over the subsequent 6 weeks of monitoring.
Allergen challenge offers another model of airway inflammation; what effect does it have on exhaled NO concentrations. In a laboratory bronchial challenge using inhaled allergen to which patients were skin-test sensitive, a rise in exhaled NO was observed in those patients who experienced a late-phase response (associated with airway inflammation) but not those who had only an early-phase response (associated primarily with smooth muscle constriction). Italian investigators monitored lung function and exhaled NO in 21 pediatric patients who were allergic to grass pollen before, during, and after their allergy season. Their exhaled NO values rose significantly during their allergy season and fell again thereafter, even though their lung function (as measured by their FEV1) remained unchanged throughout the observation period. These observations point to the possibility that one be measuring two distinct phenomena with spirometry and exhaled NO, one assessing airway caliber, the other airway inflammation.
Exhaled NO as a tool for patient management?
Two studies have been performed to address the clinically important questions: “Do exhaled nitric oxide levels predict the clinical response to steroid therapy, and can they help us to adjust the dose of corticosteroids in our patients?” The first study examined the response to oral corticosteroids (prednisolone 30 mg daily for two weeks) among 37 asthmatic patients, all but one of whom were taking inhaled corticosteroids. Measurements of their exhaled NO and sputum eosinophilia were made before initiation of systemic steroids. Despite their use of inhaled steroids, many patients still had elevated levels of exhaled NO (and of sputum eosinophils) upon initiation of systemic steroids. Nonetheless, neither marker proved sensitive in predicting a good response to prednisolone. The investigators used a cut-off of 10 ppb of exhaled NO to distinguish potentially high vs. low levels of airway inflammation. A level of exhaled NO greater than 10 ppb was only 59% sensitive in predicting clinical improvement with systemic steroids. Neither it nor sputum eosinophilia proved to be a clinically useful predictor of steroid-responsiveness in this study.
Finally, in a recent report investigators explored whether exhaled NO levels were useful in predicting loss of asthma control in a group of patients withdrawing from inhaled steroids. These 78 patients underwent carefully controlled steroid dose-reductions, with symptom diaries, home peak flow monitoring, and weekly assessments of FEV1. According to predefined criteria for loss of control, 60 patients (78%) experienced loss of control. Their initial exhaled NO value, their exhaled NO value at the visit immediately preceding their asthma flare (“penultimate visit”), and the rise in their exhaled NO value between the initial and the penultimate visit all failed to predict in a clinically useful way which patients would experience loss of asthma control.
At the present time, exhaled NO measurements may play some role in diagnosing asthma, but there is little evidence that monitoring exhaled NO levels in our asthmatic patients can help to direct our therapy. We know that NO is linked to airway inflammation, but we don’t know the mechanism for this association. Future studies will be needed to clarify the physiologic role of NO in asthma and to determine how to make its measurement clinically useful in patient management.
References:
- Massaro AF, Gaston B, Kita D, et al. Expired nitric oxide levels during treatment of acute asthma. Am J Respir Crit Care Med 1995; 152:800-3.
- Little SA, Chalmers GW, MacLeod KJ, et al. Non-invasive markers of airway inflammation as predictors of oral steroid responsiveness in asthma. Thorax 2000; 55:232-4.
- Jones SL, Kittelson J, Cowan JO, et al. The predictive value of exhaled nitric oxide measurements in assessing changes in asthma control. Am J Respir Crit Care Med 2001; 164:738-43.
About the author: Dr. Anthony Massaro is a member of the Pulmonary and Critical Care Division at the Brigham and Women’s Hospital. He is also a member of the Thoracic Oncology Program and Medical Director of the Pulmonary Function Laboratory at the Dana Farber Cancer Institute.
In the next issue: Introduction to the Pharmacogenetics of Asthma by Jeffrey Drazen, M.D.