Partners Asthma Center Grand Rounds
Albert L. Sheffer, M.D.
Fatal Asthma
Asthma mortality is increasing worldwide, yet fatal asthma is preventable. Failure to recognize the severity of asthma and failure to respond with sufficiently intensive treatment (underdiagnosis and undertreatment) are common causes of asthma deaths. A number of studies have reviewed fatal and near-fatal episodes of asthma on a case-by-case basis and concluded that 65-85% of cases could have been prevented. Often patients fail to perceive the severity of their disease; equally often health care providers underestimate the gravity of the patient's condition. Patients with severe persistent asthma are at greatest risk for life-threatening attacks, but all patients are at some risk. Fatal attacks can evolve over minutes to hours; vigilance on the part of both patient and provider is key to fatality prevention in asthma.
Epidemiology
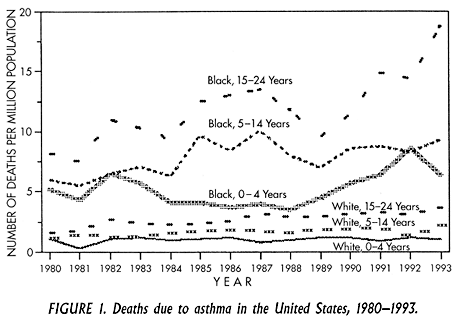
Over the past two decades, asthma mortality has increased worldwide in both developed and underdeveloped nations. In the United States, between 1980 and 1993 the number of deaths due to asthma increased by 118% (Figure 1). More recently, asthma mortality in the U.S. has plateaued at 5,000-6,000 deaths/year. Death rates are particularly high for African Americans and Hispanics (4-6 times higher than for whites) and are concentrated in urban areas such as New York City, Chicago, and Los Angeles. Multiple factors may contribute to the increased asthma mortality among inner-city minority persons, including poor access to medications and to appropriate health care, bad living conditions, high prevalence of cigarette smoking, and lack of education about disease prevention. Predictably, asthma morbidity and mortality are closely linked to poverty.
It is estimated that the risk of death from asthma is 1 in 10,000 persons with asthma. Among persons with severe disease, the risk increases to 1-3 per 100 persons. Any person who has suffered an episode of respiratory failure due to asthma has a risk of 1-2/10 persons of dying from asthma over the next 10 years.
Among inner-city children with asthma, allergen sensitivity plus exposure identifies a subgroup with more severe disease. The National Cooperative Inner-City Asthma Study investigated allergens in the bedroom of more than 400 children. Intense cockroach exposure was found in as many as 89% of the homes, and cockroach sensitivity (as demonstrated by positive skin tests to cockroach antigen) was present in approximately one third of the children. The 20% of children who had both sensitivity and intense environmental exposure to cockroach antigen had the most severe asthma: they had the highest prevalence of hospitalizations, days with wheezing, unscheduled doctor visits, and change in plans of the caregivers due to asthmatic exacerbations. In more affluent homes, dust mite, cat, and dog antigens play more prominent roles, but the impact is the same: intense antigenic exposure combined with allergic sensitivity predisposes to severe asthma and increased risk of death from asthma.
In Rochester, Minnesota, Dr. O'Halloren identified a seasonal variability in asthma deaths among young asthmatics. The fall predominance was linked to mold (alternaria) sensitivity and increased mold exposure in the fall season. By contrast, among elderly persons with asthma, deaths are most common between November and February and are probably most closely linked with an increased seasonal incidence of respiratory tract infections.
Physiology
It is possible that persons with fatal asthma have not only more severe disease than those with non-fatal asthma but also a unique physiologic abnormality that predisposes to fatal outcomes. One study of persons who survived near-fatal asthmatic attacks found a reduced perception of hypoxemia among this group of patients compared with persons with asthma who had not had near-fatal events and with normal individuals. This blunted hypoxic response suggests that during life-threatening asthmatic attacks, this subgroup of patients might experience less intense dyspnea and might therefore be more likely to delay in seeking medical attention.
Another hypothesis for which there is experimental support is that increased bronchial hyperresponsiveness (that is, very low concentration of methacholine can cause significant airflow obstruction) combined with reduced bronchial elasticity causes increased susceptibility to fatal asthma. Intense peribronchial inflammation with thickening of the adventitia causes a relative uncoupling of the bronchial wall from surrounding lung parenchyma. Lung elastic recoil is not transmitted as fully to the airways, predisposing to airway closure. Airway closure can be suspected by the finding of reduction in the forced vital capacity and confirmed by special techniques such as nitrogen wash-out curves, SPECT scans, and high-resolution CT scans. Distinctive pathologic changes in the airways of patients with fatal asthma (to be described below) may account for the changes in airway compliance that then predispose to narrowing and collapse.
Finally, some investigators have looked to abnormalities in airway smooth muscle itself that might predispose to fatal outcomes in asthma. Shortening of airway smooth muscle beyond what is normally found in its length-tension behavior, referred to as a "latchbridge" state of the interlocking actin and myosin molecules, may occur as the result of intense mediator stimulation, low tidal breaths, and lack of beta-adrenergic stimulation. Once airway smooth muscle has contracted in this way, dilation is more difficult than usual to achieve.
Pathology
In 1996 Dr. Carroll and colleagues performed detailed autopsy studies on patients with fatal asthma and on two comparison group: persons with asthma dying of other causes and a control group without asthma. Significant differences found in the fatal asthma group included significant expansion of the airway wall outside of the basement membrane (submucosal and adventitial zones). Cellular infiltration (predominantly with eosinophils), mucous gland hypertrophy/hyperplasia, and smooth muscle hypertrophy all contributed to significant airway wall thickening, most prominent in the larger airways.
This airway wall thickening can contribute to life-threatening asthmatic attacks in two ways. First, the pressure exerted by the elastic recoil of surrounding lung parenchyma to hold airways open is dissipated across this expanded peribronchial space, as discussed above. Second, any amount of bronchial smooth muscle contraction leads to greater narrowing of the airway lumen when the airway wall inside of the smooth muscle is increased in its thickness.
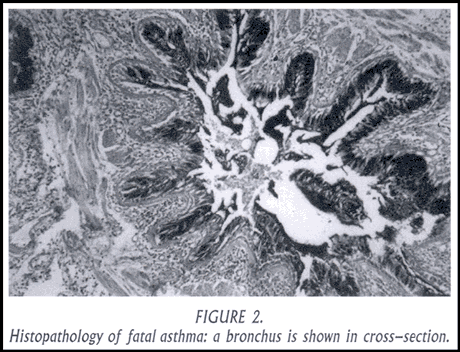
The other characteristic finding in patients dying of asthma is extensive desquamation of the epithelial lining cells and mucus hypersecretion with formation of intraluminal mucous plugs (Figure 2). It is easy to visualize that intraluminal obstruction by mucus and cellular debris combined with dynamic contraction and collapse of airways can lead to inadequate levels of ventilation and ultimately to asphyxiation and death. Pathologic studies of fatal asthma suggest that the inflammatory changes within the airway lumen and throughout the airway wall are simply more extensive and intense that in non-fatal asthma. Some of these inflammatory changes are chronic, the consequences of long-standing allergic inflammation of the airways; others are acute, a manifestation of the terminal event (sometimes infectious, other times allergic or non-specific irritant). Most of these findings are reversible with appropriate anti-inflammatory therapy. The necessity for systemic anti-inflammatory therapy in this setting is emphasized by the need to deliver medication to obstructed or collapsed airways and to the bronchial submucosa and adventitial, deep from the mucosal surface where inhaled anti-inflammatory medication is deposited.
Pharmacology
For many years there has been speculation that the medications used to treat asthma, particularly beta-adrenergic agonists, might contribute to excess mortality from asthma. Two epidemics of asthmatic deaths, one in England in the 1960s and one in New Zealand in the 1970s, were closely linked to the rise in sales of particular inhaled beta-agonist preparations: isoproterenol forte (250 µg/puff) in England and fenoterol (200 µg/puff) in New Zealand. In both instances, asthmatic deaths fell soon after withdrawal from the market of these beta-agonist preparations.
Others have related the frequency of use of beta agonists to adverse outcomes in asthma. A double-blind, cross-over study of fenoterol in stable asthma compared regular (four times daily) use of fenoterol with intermittent (as needed) use. Almost all outcomes were worse during the six-month period of regular fenoterol use, including daytime symptoms, nocturnal symptoms, frequency of night-time bronchodilator use, and need for courses of oral prednisone.
An association between beta agonist use and death from asthma was demonstrated in a large retrospective study from Saskatchewan, Canada. The number of prescriptions filled for inhaled beta agonists, including albuterol as well as fenoterol, were compared between 44 patients with fatal asthma and several hundred control patients, among whom an attempt was made to match disease severity. These investigators found a dose-effect relationship between prescriptions for canisters of beta-agonist bronchodilator filled within the preceding year and risk of death from asthma. The relative risk of death from asthma was an astonishing 32:1 among persons filling prescriptions for 25 or more albuterol inhalers in the preceding year (or averaging use of just over 2 canisters per month). This study does not, of course, establish any causal relationship between beta-agonist use and fatal asthma, but it does indicate a need to explore potential mechanisms whereby asthma pharmacology and asthma deaths might be related.
A recent study in this regard explored the effect of regular beta agonist use on the bronchoprotective effect of beta agonists. Among a small group of persons with mild, stable asthma, subjects were randomly assigned to use either albuterol or placebo 2 puffs four times daily; both groups could use as-needed albuterol for "rescue." Although the bronchodilator effect of albuterol was not changed by regular use of albuterol, the bronchoprotective effect of albuterol was reduced. Albuterol did not blunt methacholine- or allergen-induced bronchoconstriction as effectively after regular beta-agonist inhalation for two weeks as it did in the placebo group. Tolerance to the bronchoprotective effect of beta agonists may be one way in which overuse of inhaled beta agonists might contribute to fatal outcomes in asthma.
Molecular studies of the beta-agonist receptor have revealed polymorphisms in the general and asthmatic populations. Some genetically determined beta-receptor types may be more susceptible to phosporylation and thereby to down-regulation, a potential mechanism for the development of tolerance to the bronchoprotective effects of beta agonists. An estimated 5-10% of the population have this particular phenotypic expression of their beta receptor and may as a result be at increased risk for severe, potentially fatal asthma.
The message here is not that beta agonists are dangerous. Used properly, they can be life- saving. The key point is that their role in asthma is as rescue medication for relief of acute asthmatic symptoms. We do no longer prescribe albuterol for regular use on a Q.I.D. basis. Frequent use of albuterol (more than one cannister [=200 puffs]/month should be a warning sign for poorly controlled asthma requiring an intensification of anti-inflammatory therapy.
Clinical Aspects
A number of clinical characteristics, some related to asthma and others related to the patient's general physical and mental condition, can help to identify a patient at increased risk for dying from asthma. These include the presence of severe, persistent asthma; large swings in peak expiratory flow (reflecting exaggerated bronchial hyperresponsiveness); prior history of respiratory failure due to asthma; recent hospitalization and recent dose reduction of prednisone; poor access to emergency health care facilities; and depression or other psychologic states that interfere with effective chronic disease management (Table 1). Family disturbances, abnormal reactions to separation, and an exaggerated sense of helplessness and despair are all psychologic states that have been associated with fatal asthma.
Table 1
|
|
Also, certain physician behaviors in treating asthmatic attacks are associated with bad outcomes. Specifically, underrecognition of the severity of an attack and underuse of systemic corticosteroids to treat severe attacks have been identified as contributing to asthmatic deaths. Use of lung function measurements, such peak flow monitoring or spirometry, at every office visit with an asthmatic patient can help to avoid missed diagnoses and underestimations of disease severity.
Patient education about asthma and its management is a crucial element of fatality prevention. Good asthma care includes time spent with our patients to encourage regular use of preventative medications, to discourage overreliance on the short-acting inhaled beta-agonist bronchodilators, and to emphasize allergen avoidance and environmental control measures tthat will reduce exposure to the inciters of asthmatic attacks.
Good care also involves teaching patients how to respond appropriately to an asthmatic attack. This point was brought home by a study conducted in Australia that compared characteristics of patients suffering fatal asthmatic attacks versus patients who recovered following near-fatal events. The two groups appeared comparable in most aspects, including prior severity of their asthma, number of hospital admissions within the preceding year, number of patients who had previously suffered episodes of respiratory failure, and availability of peak flow meters for home use. What distinguished the two groups, these authors found, was the increased delay in seeking and receiving medical care among the group with fatal outcomes. Others have commented on the barriers to seeking timely care for severe asthmatic exacerbations. These include blunted perception of dyspnea, disregard of symptoms (perhaps recalling other severe asthmatic attacks in the past that eventually got better with self-management; perhaps being unaware that asthma can lead to fatal asphyxiation), poor self-management skills (such as relying on the temporary symptomatic improvement derived from the inhaled bronchodilators), and deficient family supports. Some of these barriers can be overcome by teaching patients to monitor their lung function at home with peak flow meters and to respond to low values (peak flow <50% or normal or of the individual's personal best value) with their individualized asthma "Action Plan." A written action plan, discussed with the patient and available to him/her at the time of an asthmatic crisis, can be an invaluable aid.
Most asthma fatalities are the culmination of deteriorating lung function that occurs over a period of several days. In a minority of cases, the attacks appear to have evolved over a very brief time span, perhaps over minutes to only a few hours. There is some evidence that these "hyperacute" attacks represent a distinct entity: pathologic studies have found a predominance of neutrophils in the airways, as opposed to the usual eosinophilia. Other investigators have argued that the observed airway neutrophilia may simply represent the earliest phase of an allergic response that with time would have evolved into the typical eosinophilic bronchitis. Persons who can develop very rapidly life-threatening airways obstruction pose the greatest challenge for fatality prevention. Constant vigilance, a readily available, epinephrine-containing pre-filled syringe with needle (Epi-Pen®), and an emergency plan shared with family and friends are important elements of care for persons with hyperacute attacks.
References:
1. Sheffer AL, ed., Fatal Asthma, in series Lung Biology in Health and Disease, Lenfant C, exec. ed., Marcel Dekker, N.Y., 1998.
2. Weiss KB, Wagener DK. "Changing pattern of asthma mortality: identifying target populations at risk." JAMA 1990; 264:1683-7.
3. Carrol N, Elliot S, Morton A, et al. "The structure of large and small airways in nonfatal and fatal asthma." Am Rev Respir Dis 1993; 147:405-10.
About the author: Dr. Albert Sheffer is Clinical Professor of Medicine at Harvard Medical School and former Director of Allergy at Brigham and Women's Hospital. He has served as chairman of the National Asthma Education and Prevention Program and of the Global Initiative on Asthma sponsored by the National Institutes of Health and the World Health Organization. He is editor of a scientific text called Fatal Asthma, published by Marcel Dekker as part of its Lung Biology in Health and Disease series.