Partners Asthma Center Grand Rounds
Ann J. Woolcock, M.D.
What is Asthma?
There is as of yet no fully satisfactory definition of asthma. Depending on whether you are a clinician, pathologist, physiologist, cell biologist, or epidemiologist, you will view asthma differently.
A clinician's definition of asthma is relatively straightforward: symptoms of cough, wheeze, and/or chest tightness that occur episodically (spontaneously or in response to provocative stimuli) and are relieved by bronchodilator drugs. The problem with this definition becomes manifest when one tries to do comparative studies among different groups of patients and investigators. This definition relies on memory, on language, and on patients' perception of symptoms. One example of the lack of precision of this definition comes from the International Study of Asthma and Allergy in Children (ISAAC), which attempts to define the prevalence of asthma in different countries worldwide. When asked whether their child had wheezing within the last 12 months, 30% of parents in Australia, New Zealand, and the United Kingdom responded "yes" (as did 25% of parents in the United States and Canada). However, when asked the same question after viewing a videotape that displayed wheezing children, the parents' affirmative responses fell to 18% and 15%, respectively.
A pathologist's definition of asthma is airway inflammation characterized by eosinophils and thickening of all layers of the airway wall, including the basement membrane. The problem with this definition is that the evolution of these inflammatory changes over time and the relationship between the inflammation and asthmatic symptoms are unknown. Pathologists do not have the opportunity to make serial observations throughout the course of asthma, especially among persons with mild disease.
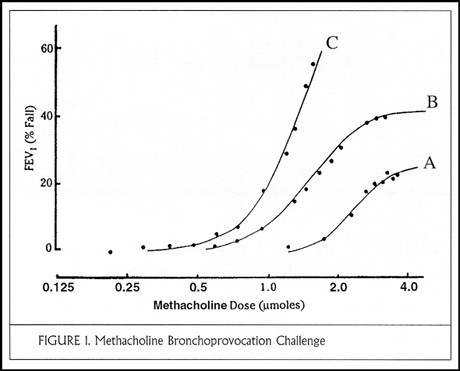
The physiologist defines asthma as airways that narrow too much and too easily (that is, hyperresponsive airways), causing airway closure and symptoms. The problems here are that not all airway hyperresponsiveness is asthma, and the measurement of airway responsiveness with methacholine or histamine is imprecise. Methacholine dose-response curves are displayed in Figure 1, with incremental doses of methacholine (expressed in micromoles or mg/ml) on the abscissa and change in FEV1 on the ordinate. In a normal curve (curve A) the FEV1 decreases by less than 20% even at the highest dose of methacholine (4 micromoles or 8 mg/ml). Curve B demonstrates mild hyperresponsiveness and has a plateau at the highest doses; this patient is probably safe from further airways obstruction. By contrast, curve C is illustrative of severe hyperresponsiveness; a small dose of methacholine causes the FEV1 to fall by 20% and no plateau is demonstrated at higher doses. This patient is at risk for life-threatening airways obstruction. The aim of asthma therapy is to shift these curves toward the right.
Calculation of the Dose-Response Ratio
One can express these relationships in terms of the dose-response ratio: the observed fall in FEV1 divided by the total micromolar dose of methacholine that caused it. A normal value is approximately 4 or less. Severe hyperresponsiveness is characterized by dose-response ratios on the order of 100 or more.
The cell biologist's definition of asthma is an allergic reaction of the airways driven by T helper lymphocyte type 2 (TH2) cells with involvement of IgE antibody and many inflammatory mediators, cytokines, and transcription factors. But how does the allergic reaction cause symptoms, and why does it do so only in some of the people with atopy? Over the last two decades, a virtual industry of research laboratories has been created to study the role of airway inflammation in asthma, spurred in part by the dramatic benefit derived from antiinflammatory therapy, particularly the inhaled corticosteroids. To some extent, this focus has come at the cost of ignoring the airway smooth muscle cell, about which we will speak more later.
The Epidemiologist's Definition of Asthma
The epidemiologist views asthma as recent symptoms (chest tightness or wheeze) plus airway hyperresponsiveness. It is difficult, however, to define precisely the cut-off point for abnormal airway responsiveness (unless you use the dose-response ratio method). In Australia we collected data on a large number of school children to assess the prevalence of asthma. We performed allergy skin tests using 8 common allergens to determine atopy, and 35-45% of children had positive reactions. We asked parents whether the child had had wheezing within the previous 12 months, with approximately 30% affirmative responses. Of these, most but not all were allergic by skin testing.
We also performed histamine provocation challenges in these children to test for airway hyperresponsiveness. Where a history of recent wheezing and bronchial hyperresponsiveness overlap, one has current asthma. In fact, this group has persistent asthma, and they tend to remain in this category as they grow older. Note that most of these children (88%) are allergic.
What about children with airway hyperresponsiveness but no symptoms; do they have asthma? And what about those children with wheezing, half of whom are allergic, with normal airway responsiveness? I suspect that at least some of them have intermittent asthma.
Ten years later, in 1992, we repeated the same set of assessments in another cohort of school children living in the same environment and attending the same schools. Although the percentage of allergic children remained the same, we observed large increases in the presence of symptoms (recent wheezing), in airway hyperresponsiveness, and in the area of overlap defined as current asthma. There is little doubt that this represents a true increase in the prevalence of childhood asthma in Australia.
We also collected sequential measurements of lung function and airway hyperresponsiveness in the original cohort of children followed for 10 years. The group of children originally having current asthma continued to have asthma through age 20. Their airway hyperresponsiveness (histamine dose-response ratio) decreased slightly at age 15 but then at age 20 returned back to approximately 60. The group that originally had wheezing but normal airway responsiveness did not develop airway hyperresponsiveness on follow up. Similarly, the group that originally had asymptomatic airway hyperresponsiveness continued asymptomatic; their dose-response ratios decreased slightly at age 15 but returned to the abnormal range at age 20. Their airway hyperresponsiveness is probably too mild to cause symptoms.
When the original cohort of children were 20 years old, we had the opportunity to study their concentration of exhaled nitric oxide (NO), a marker for asthmatic-type airway inflammation. We compared those with current asthma, those with asymptomatic airway hyperresponsiveness, those with wheezing but normal airway responsiveness, and normal children. The group with current asthma had highest levels of exhaled NO. We found an intermediate level in those with asymptomatic airway hyperresponsiveness. Interestingly, the children with wheezing but normal airway responsiveness had levels indistinguishable from normal.
Airway Hyperresponsiveness Distinct from Airway Inflammation
The next question that these observations call to mind is whether airway hyperresponsiveness is due entirely to airway inflammation or whether other abnormalities in the airways may also be responsible for the disease that we call asthma. I began to consider this question in the 1980s, based on an observational study performed by one of my fellows. We treated a group of asthmatic patients with inhaled beclomethasone 800 µg/day for several years and made serial measurements of their airway responsiveness. This was not a controlled trial, and we were unable to address the issue of medication compliance. Nonetheless, what we found was that even after an average of 4 1/2 years of treatment, airway responsiveness was well controlled in only approximately one third of patients. Airway responsiveness remained moderate in another third and severe in the remaining third of patients. One would have thought that if airway responsiveness were due exclusively to inflamed airways that long-term administration of inhaled steroids would have completely corrected the problem.
Then in 1995 Dr. Noe Zamel went to the island of Tristan da Cunha, with its genetically homogeneous population and known high prevalence of asthma, and measured atopy and airway responsiveness in its entire population over the age of 5 (approximately 300 persons). He found a prevalence of atopy (47.6%) not different from other adult populations around the world. However, the prevalence of recent wheezing was slightly higher and the prevalence of airway hyperresponsiveness dramatically higher than in Australia, giving a remarkable 23% prevalence of persistent asthma on the island. There is some overlap between airway hyperresponsiveness and atopy, suggesting that allergic airway inflammation contributes to some of the hyperresponsiveness. However, the unusually high prevalence of airway hyperresponsiveness on the island, including hyperresponsiveness in the absence of atopy, suggests an inheritance of airway hyperresponsivenes that may be different from atopy.
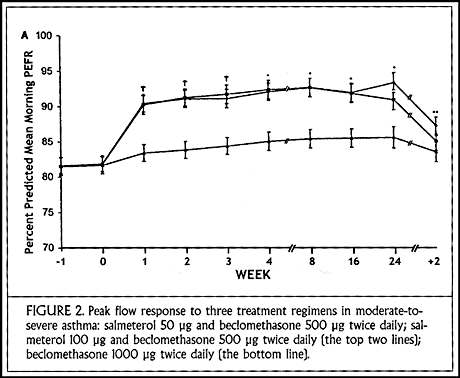
My thinking on this subject was also greatly influenced by an international study that we conducted using the long-acting inhaled beta agonist, salmeterol. Patients with moderate-to-severe asthma still symptomatic despite taking beclomethasone 500 µg twice daily were randomly assigned either to double their daily dose of inhaled corticosteroids or to add salmeterol in one of two different doses (50 or 100 µg twice daily). There were no differences between the two salmeterol doses.
Patients randomized to receive salmeterol had an immediate improvement in morning peak flow values that continued for the duration of the study (24 weeks) (Figure 2). There was slow improvement over 6 months in the group treated with high-dose inhaled steroids, and it is possible that they might have caught up to the other groups over the course of 1-2 years. Most striking in the salmeterol-treated groups was the reduction in nocturnal awakenings due to asthma. By the end of the first week of treatment, nearly 100% experienced symptom-free nights.
In our experience, patients treated with salmeterol repeatedly report how much better they feel, having slept all night and waking feeling well. In Australia it is mandated that salmeterol be used only in conjunction with inhaled steroids, never as monotherapy for asthma.
To summarize, there are a number of lines of evidence, some of which I have reviewed with you today, that indicate that there are two abnormalities in asthma and that asthma is not just airway inflammation. 1) Not all persons with allergies have wheezing symptoms. 2) Airway hyperresponsiveness seems to be inherited separately from atopy. 3) In animal models, it has clearly been shown that airway inflammation can be separated from airway hyperresponsiveness. 4) Following long-term treatment with inhaled corticosteroids, when airway inflammation is no longer evident by biopsy or analysis of bronchoalveolar lavage fluid or induced sputum analysis or measurement of exhaled NO, one can still demonstrate airway hyperresponsiveness. 5) Use of long-acting bronchodilators in combination with low doses of inhaled steroids has proved more effective than high doses of inhaled steroid for treatment of asthma.
Airway Smooth Muscle Dysfunction in Asthma
What might cause airway hyperresponsiveness independent of airway inflammation? Work by Dr. Jeffrey Fredberg and others at the Harvard School of Public Health has pointed us toward consideration of abnormal behavior of the airway smooth muscle. Normally, airway smooth muscle contracts against an elastic load imposed by the recoil forces of surrounding lung tissue. Among persons with airway hyperresponsiveness, one finds abnormal unloading of airway smooth muscle for some reason. Normal stretches of the muscle imposed by tidal breathing do not keep the muscle from having too much tone. One might use this concept of an independently inherited abnormality of airway smooth muscle unloading to explain the epidemiologic observations that we have discussed. Persons who inherit a mild allergic tendency but no abnormality of smooth muscle might have positive skin tests (atopy) but not airway hyperresponsiveness and no asthmatic symptoms. If one inherits severe seasonal allergies, for example, you might have rhinitis or even seasonal asthma due to airway inflammation. On the other hand, inheritance of a mild abnormality of airway smooth muscle function in the absence of atopy may account for the group of persons who have airway hyperresponsiveness without any allergic tendency or wheezing. If the inherited abnormality of airway smooth muscle function is severe, one may get non-allergic ("intrinsic") asthma. The overlap of both atopy and abnormal airway smooth muscle unloading is classic allergic asthma.
For the moment, then, my current definition of asthma is as follows:Unloading of the airway smooth muscle — usually associated with airway inflammation — that causes the airways to narrow too easily and too much, resulting in wheeze and chest tightness. It responds to adequate doses (diligently applied) of inhaled corticosteroids, but long-acting inhaled beta agonists may be needed until the airway smooth muscle becomes dynamically loaded.
References:
The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema. Lancet 1998; 351:1225-32.
Peat JK, van den Berg RH, Green WF, et al. Changing prevalence of asthma in Australian children. BMJ 1994; 308:1591-6.
Zamel N, McClean PA, Sandell PR, et al. Asthma on Tristan da Cunha: looking for the genetic link. Am J Respir Crit Care Med 1996; 153:1902-6.
Woolcock A, Lundback B, Ringdal N, Jacques LA. Comparison of addition of salmeterol to inhaled steroids with doubling of the dose of inhaled steroids. Am J Respir Crit Care Med 1996; 153:1481-8.
About the author: Dr. Ann J. Woolcock is Professor of Respiratory Medicine at the University of Sydney, Australia, and Director of the Institute of Respiratory Medicine at the Royal Prince Alfred Hospital in Sydney. She is an internationally renowned authority on the epidemiology, physiology, and management of asthma.